Quality of Chaetoceros calcitrans Cultured with Different Concentrations of Potassium Nitrate (KNO3)
on
Advances in Tropical Biodiversity and Environmental Sciences 4(1): 5-9, February 2020 e-ISSN:2622-0628
DOI: 10.24843/ATBES.2020.v04.i01.p02 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/57607
5
Quality of Chaetoceros calcitrans Cultured with Different Concentrations of Potassium Nitrate (KNO3)
Alfarisi Prafanda*, Pande Gde Sasmita Julyantoro, Ni Putu Putri Wijayanti
Department of Aquatic Resources Management, Faculty of Marine and Fisheries, Udayana University Jl. Kampus Unud Bukit Jimbaran, Kuta Selatan, Badung, Bali *Corresponding author: alprafanda@gmail.com
Abstract. Addition of different fertilizer concentrations into cultivation media affects the cell density of microalgae. Potassium nitrate (KNO3), is one of the components in Guillard fertilizer composition commonly used for microalgae culture. This study aims to determine the quality of Chaetoceros calcitrans when cultured with different concentration of KNO3. This research was conducted from November 2019 to January 2020 at Balai Perikanan Budidaya Air Payau (BPBAP) Situbondo and Biosain Laboratory, Jember State Politeknik, East Java. This study consists of four treatments and three replicates. The object in this study was C. calcitrans culture with initial density 105 cells/ml. The main parameters observed were cell density, biomass, protein and amino acids contents and medium parameters such as temperature, pH, DO, salinity, nitrate and phosphate. There were four concentrations of KNO3 used in this study, namely control (75 g/l), treatment Group A (100 g/l), treatment B (125 g/l) and treatment C (150 g/l). The results showed that the cells density of C. calcitrans at control, treatment Group A, B and C were 110.4; 105.2; 108.3; and 100.8 (×104 cells/ml), respectively. This study indicated that different concentration of KNO3 affect the starting point of stationary phase, but One Way ANOVA test showed that those treatments had no significant effect (P≥0,05) on the growth rate and dry biomass of C. calcitrans. Finally, we found that the protein content in addition of 75, 100, 125 and 150 g/l KNO3 were 9.748; 8.802; 6.812; and 3.776%, respectively.
Keywords: Culture Quality; Chaetoceros calcitrans and KNO3
I. INTRODUCTION
Feed quality is one of key success factors in aquaculture production, especially in larviculture phase. One of natural live feed for fish larvae that contains high nutrient content is Chaetoceros calcitrans. This microalga is classified as phytoplankton which is easy to culture and needs short time (3-5 days) to growth. C. calcitrans is 23 species of natural live feeds commonly used for fish or shrimp larviculture. The requirements of Chaetoceros sp. to supply the live feed demand for shrimp larvae normally ranged between 20.000 – 120.000 cell/ml, which vary amount for each growth stage of shrimp larvae [1].
The growth of C. calcitrans is influenced by the availability of nutrient in media and its surrounding environments [2]. The composition of nutrients with certain concentration affected the production of biomass and nutrient of microalgae. One kind of nutrient that commonly added to cultivation media is Potassium nitrate (KNO3). Potassium nitrate is ionic compound arranged by cation K+ and NO3- and it is the most important source of nitrogen [3].
Nutrient content of Chaetoceros sp. is influenced by the condition of cultivation media, both of physical and chemical parameters. Generally, the growth of microalgae is affected by some factors such as light, nutrient,
cultivation age and others environmental factors. The content of active anti-microbials component from Chaetoceros sp. which is cultivated with difference duration exposed of radiation, is known to have different growth pattern and biomass yields [4]. However, the growth pattern and the value of protein content of C. calcitrans cultured using different concentration of nutrient has not been much explored. Therefore, this research is important to conducted for determining the quality of C. calcitrans culture with addition of different concentration of KNO3
II. RESEARCH METHODS
Experimental set up
This research uses experimental design. C. calcitrans inoculant was used with initial density of 105 cell/ml. This research consisted of 4 treatments with 3 replicates for each treatment.
Materials and Media Sterilization
Eighty liters container used in this study was cleaned and washed prior to use. This container was filled with seawater and then 10 ppm chlorine was added for sterilization. It was then aerated for 24 hours. After 24 hours, the seawater for cultivation media was neutralized by adding sodium thiosulfate and aerated for 2 hours.
Fertilizer for C. calcitrans culture
The ingredients of the fertilizer were 75, 100, 125 and 150 g/l of KNO3, 5 g/l NaH2PO4, 3.15 g/l FeCl3 and 5 g/l Na2EDTA. These ingredients were added and mixed by stirrer to 1000 ml sterile aquadest. Fertilizer solution put into 1-liter erlenmeyer and sterilized using autoclave. The composition of Guillard fertilizer-used in this research can be seen in Table I.
TABLE I
COMPOSITION OF FERTILIZER
|
Composition |
Treatment | |||
|
Control |
A |
B |
C | |
|
Guillard Fertilizer | ||||
|
KNO3 |
75 g |
100 g |
125 g |
150 g |
|
NaH2PO4 |
5 g |
5 g |
5 g |
5 g |
|
Na2EDTA |
5 g |
5 g |
5 g |
5 g |
|
FeCl3 |
3,15 g |
3,15 g |
3,15 g |
3,15 g |
|
Silicate Silicate |
30 g |
30 g |
30 g |
30 g |
|
Vitamin B1 |
100 mg |
100 mg |
100 mg |
100 mg |
|
B12 |
5 mg |
5 mg |
5 mg |
5 mg |
The Preparation of C. calcitrans Inoculant
C. calcitrans inoculant was obtained from live feed laboratory of BPBAP Situbondo.The inoculant C. calcitrans was collected during exponential growth phase. The inoculant was added into cultivation media at 105 cell/ml initial density.
Cultivation and Cell Density Calculation of C. calcitrans
The Guillard fertilizer, silicate and vitamin were added as much as 1 ml/l to sterilize seawater for cultivation media. Inoculant was poured and keep aerated for oxygen supply. The calculation was done by calculating the amount of cell using hemocytometer for each treatment every 8 hours, regularly. The equations described below:
A1 + A 2 + A3 + A 4 + A5
N =-------------------x104
5
Noted: N is Cell density (sel/ml), A1-A5 is cell density in boxes 1-5, 5 is total of hemocytometer boxes
observed, and 104 is box volume.
Water Quality Parameter
Water quality parameters in this research namely pH, temperature, DO, salinity, nitrate (NO3), and phosphate (PO4). The measurement pH, temperature, DO, and salinity were done in every 8 hours in situ, while the nitrate and phosphate were analyzed ex situ in fish and environmental health laboratory, BPBAP Situbondo.
The Calculation of Biomass
The analysis of C. calcitrans biomass was performedwhen harvested after 112 hours culture period.
-
C. calcitrans was harvested at the beginning of stationer phase as the peak growth period of microalgae culture.
The Analysis of Protein and Amino Acid Content
The analysis of protein and amino acid content of C. calcitrans cultured with different concentration of KNO3 was conducted in Bioscience laboratory of Jember State Politeknik. The protein content was analyzed using Kjehdahl method which consist of three phases such as destruction, distillation, and titration. Meanwhile amino acid analysis was done using LC-MS (Liquid Chromatography-Mass Spectrophotometry).
Data Analysis
The growth and dry biomass data obtained in this research was analyzed using variance analysis or One Way Analysis of Variance (ANOVA) with 5% significance level. When the data variance of adding different concentration of potassium nitrate (KNO3) resulted statistically significant difference, the test was continued by Duncan post hoc test.
-
III. RESULT AND DISCUSSION
The Growth and Dry Biomass of C. calcitrans
The result showed that the C. calcitrans was grown for 112 hours. The result from microalgae’s harvest in stationer phase showed that C. calcitrans cultivated using 75 g/l KNO3 has the highest cell density of 1.1 × 106 cell/ml. The lowest cell density was obtained in C. calcitrans’s cultivation with 150 g/l of KNO3 fertilizer about 94.3 × 104 cell/ml.
The growth phase of C. calcitrans cultivated using different dosage of KNO3 resulted in different time interval of each growth phase. Time interval difference in lag phase of each treatment might be caused by difference concentration of starting nutrient component in cultivation media to support the growth of C. calcitrans microalgae. The duration of lag phase was in line with the increase of KNO3 concentrations.
The graph in Figure 1 showed that the higher of KNO3 content in cultivation media, the shorter time that microalgae needed to adapt. The difference of growth pattern also can be seen in Figure 1. In the starting point of inoculation, the growth was relatively slow. It indicated that phytoplankton was adapting with its environment. In early period of cultivation, the content of nutrient was still high that could be used by each phytoplankton to support the growth process [5]. Microalgae have different adjustments to external factors such as nutrients according to the ability of the microalgae tolerance [6]. The result of One Way ANOVA (Analysis of variance) indicated 0.994 signification score (p≥0.05) which means that there was no significant difference between the treatment with the difference
concentration of KNO3 to the cell density of C. calcitrans at the 112nd culture hour within 95% probability level (α=0,05). The density of microalgae was not significantly different might be caused by there was other factors that has more influence on the growth of microalgae, so that the difference dosage of KNO3 did not give significant
effect on C. calcitrans’ growth. The study by Kawaroe et al. [7] about the growth rate of Chlorella sp. and Dunaliella sp. based on the nutrient and photoperiod difference stated that photoperiod influence more to the growth than the concentration of nutrient.
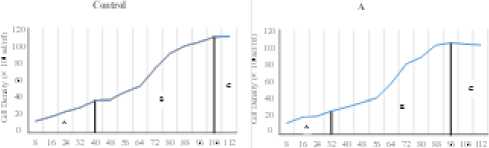
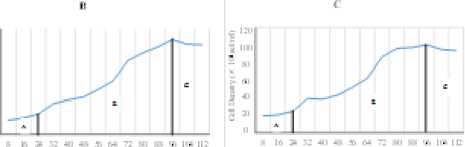
Fugure 1. Growth Pattern in Each Treatment. A = Lag phase; B = Exponential phase; C = Stationer phase
The C. calcitrans biomass in each treatment was vary. The average of the highest dry biomass was found in controlled treatment with 75 g/l of KNO3 about 8.17 g. Meanwhile, the lowest dry biomass was obtained in treatment C with 150 g/l KNO3 about 6.53 g (Figure 2).
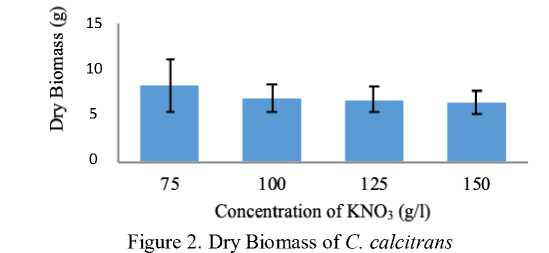
The normality and homogenity test on the dry biomass of C. calcitrans showed 0,494 signification score (p≥0.05) which means there was no significant influence on adding difference concentration of KNO3 to the dry biomass of C. calcitrans. The treatment with 75 g/l of KNO3 has a higher biomass, probably caused by the high density of the microalgae when the cultivation was in progress. It was also might be caused by proper harvest time that was near the period of stationer phase which influenced maximize produced of microalgae biomass. Trikuti et al. [8] stated that the difference of the amount of biomass and cell’s size of C. calcitrans in various media probably because of the difference availability of nutrient. The harvest of cultivated biomass was usually done in the stationer phase to make its biomass obtained the maximal amount [9].
Protein Content and Amino Acid
The average of protein content of C. calcitrans analyzed using Kjehdahl method was vary. The analysis of protein content data showed that the highest protein content obtained in cultivation treatment with 75 g/l of KNO3 about 9,748%, while the lowest was in C treatment (150 g/l of KNO3) about 3,776%. The protein content of
C. calcitrans can be seen in Figure 3.
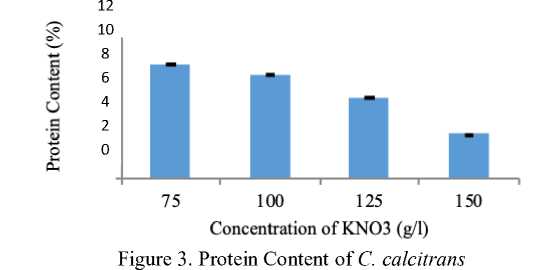
The protein content in C. calcitrans seems not in line with the amount of KNO3 concentration. The higher concentration of KNO3 in cultivation media was followed by the decrease of protein content in C. calcitrans. It might be caused by the high concentration of KNO3 was not used optimally for microalgae growth thus affecting the protein content and also probably that other nutrient components have more strong influence on the growth of C. calcitrans, such as FeCl3 with different concentrations in each type of fertilizer. The content of FeCl3 had ability to convert the nitrate became nitrite, then it converted nitrite to the form of ammonium [10].
The analysis of amino acid content in C. calcitrans which was tested using LC-MS showed various result as presented in Tabel 2. There were 16 types of amino acid tested in this study and 11 types of amino acids were detected in C. calcitrans, while other 5 amino acids were not detected. The treatment with 75 g/l of KNO3 had a slightly higher amino acid content compared to other treatments, this was thought to be related to the protein content produced. Nine essential amino acids were obtained in C. calcitrans. It is fewer compared to the result from Herawati and Hutabarat [11] which obtained 10 essential amino acids from Skeletonema costatum. The difference of amino acid content could be caused by the difference of microalgae species, nutrient availability and environment condition during the cultivation. Some
factors that influenced the growth and biochemical composition were species, light and nutrient [12].
|
TABLE II AMINO ACID CONTENT IN C. calcitrans | ||||
|
Amino Acid (%) |
75 g/l |
Treatment |
150 g/l | |
|
100 g/l |
125 g/l | |||
|
Glycine |
Nd |
nd |
nd |
nd |
|
Threonine |
0,0040 |
0,0018 |
0,0027 |
0,0033 |
|
Valine |
Nd |
nd |
nd |
nd |
|
Alanine |
Nd |
nd |
nd |
nd |
|
Glutamic Acid |
0,0026 |
0,0022 |
0,0016 |
0,0021 |
|
Lysine |
0,0208 |
0,0181 |
0,0167 |
0,0194 |
|
Tyrosine |
Nd |
nd |
nd |
nd |
|
Leusin / Isoleusin |
0,0044 |
0,0035 |
0,0035 |
0,0040 |
|
Aspartic Acid |
0,0020 |
0,0014 |
0,0018 |
0,0014 |
|
Arginine |
0,0017 |
0,0020 |
0,0017 |
0,0018 |
|
Asparagine |
0,0158 |
0,0194 |
0,0188 |
0,0190 |
|
Histidine |
0,0024 |
0,0025 |
0,0022 |
0,0024 |
|
Cystine |
0,0442 |
0,0497 |
0,0612 |
0,0586 |
|
Methionine |
0,0686 |
0,0608 |
0,0238 |
0,0581 |
|
Phenylalanine |
0,0584 |
0,0712 |
0,0561 |
0,0583 |
|
Proline |
Nd |
nd |
nd |
Nd |
|
Note: nd = not detected | ||||
Water Quality Parameter
Water quality parameter during the cultivation of C. calcitrans microalgae was in optimum level for microalgae growth. Temperature, dissolved oxygen, pH and salinity in the culture period are in the optimum range for microalgae growth [13]. Adding of various KNO3 concentration resulted in various nitrate content in cultivation media. The water quality parameter during culture period is presented in Table 3.
TABLE III
DATA CALCULATION OF WATER QUALITY PARAMETER
|
Parameter |
Treatment | |||
|
75 g/l |
100 g/l |
125 g/l |
150 g/l | |
|
Temperature (oC) |
24,9- |
24,9- |
24,8- |
24,6- |
|
29,3 |
29,2 |
29,0 |
29,1 | |
|
DO (ppm) |
5,7-6,5 |
5,5-6,5 |
5,6-6,6 |
5,6-6,5 |
|
pH |
8,0-8,7 |
8,0-8,5 |
8,0-8,6 |
8,0-8,6 |
|
Salinity (ppt) |
35 |
35 |
35 |
35 |
|
Start |
32,3 |
50,0 |
40,7 |
65,8 |
|
Nitrate (mg/l) End |
53,6 |
41,4 |
63,3 |
48,8 |
|
1,152 |
1,345 |
0,996 |
1,333 | |
|
Phosphate (mg/l) Start |
2,570 |
3,710 |
2,730 |
3,520 |
-
IV. CONCLUSION
The difference KNO3 concentration in C. calcitrans culture resulted in different time for reaching the initial of exponential phase. The higher KNO3 concentration reach faster initial exponential phase than the lower one. However, this different concentration of KNO3 have no significance different on the growth and dry biomass of C. calcitrans. Finally, the increase of KNO3 concentration
was not in line with increasing of protein content of C. calcitrans.
ACKNOWLEDGMENT
We acknowledge Balai Perikanan Budidaya Air Payau (BPBAP) Situbondo, Bioscience Laboratory of Jember State Politeknik and research team for all kind contribution to this research.
REFERENCES
-
[1] [FAO] Food and Agriculture Organization. 1996. FAO Fisheries Technical Paper.
http://www.fao.org/3/w3732e/w3732e08.htm#. Top of Page (Accessed 2nd of August 2019).
-
[2] Ermayanti E. 2011. Chemical Components of Cultivation Chaetoceros Gracilis in Outdoor Using NPSI Fertilizer. Skripsi. Bogor: Teknologi Hasil Perairan:Institut Pertanian Bogor.
-
[3] Sanjaya AS, JA Prajaka, N Aini, TH Soerawidjaja. 2017. Determination of Potassium Content in Oil Palm Empty Fruit Bunch in Langsat Area East Kutai Using Extraction Method. Jurnal Integrasi Proses, 6(4):07-12.
-
[4] Setyaningsih I, Desniar, E. Purnamasari. 2012. Antimicrobials from Chaetoceros gracilis Cultivated with Different Irradiation Times. Jurnal Akuatika, 3(2): 180-189.
-
[5] Rizky YA, I Raya, S. Dali. 2012. Determination of Phytoplankton Cell Growth Rate Chaetoceros calcitrans, Chlorella vulgaris, Dunaliella salina, and Porphyridium cruentum. Skripsi. Makassar: Kimia, Universitas Hasanuddin
-
[6] Huang WW, BZ. Dong, ZP Caidan, SS Duan. 2011. Growth Effect on Mixed Culture of Dunaliella salina and Phaeodactylum trucormutum under different inoculation densities and nitrogen concentrations. African Journal of Biotechnology, 10(61): 13164
13174.
-
[7] Kawaroe M, T Prartono, A Sunuddin, DW Sari, D Augustine. 2009. Specific Growth Rate of Chlorella sp. and Dunaliella sp. Based on the Difference between Nutrients and Photoperiods. Jurnal Ilmu-Ilmu Perairan dan Perikanan Indonesia, 16(1): 7377.
-
[8] Trikuti K, AAMD Anggreni, IBW Gunam. 2016. Effect of Media Type on the Biomass Concentration and Protein Content of Chaetoceros Calcitrans. Jurnal Rekayasa Dan Manajemen Agroindustri, 4(2): 13-22.
-
[9] Akbar TM. 2008. Effect of Light on Antibacterial Compounds of Chaetoceros gracilis. [Skripsi]. Bogor: Teknologi Hasil Perairan: Institut Pertanian Bogor.
-
[10] Wijaya SA. 2006. Effect of Different Urea Concentration on Growth of Nannochloropsis
oculata. Skripsi. Surabaya: Kedokteran Hewan,
Universitas Airlangga
-
[11] Herawati VE, J Hutabarat. 2014. Effects of Growth, Fat Content and Profile Essential Amino Acid of Skeletonema costatum in Mass Culture Using Different Technical Culture Media. Jurnal Ilmu
Perikanan dan Sumberdaya Perairan, 3(1):221-226.
-
[12] Borowitzka MA. 1988. Algal Growth Media and Sources of Algal Cultures. Cambridge: Cambridge University Press.
-
[13] Rai SV, M Rajashekhar. 2014. Effect of pH, Salinity and Temperature on The Growth of Six Species of Marine Phytoplankton. J. Algal Biomass Utln, 5(4): 55-59.
Discussion and feedback