Seagrass (Enhalus acoroides) as an Heavy Metal Bioindicator on Biomonitoring Water Quality in Sanur Beach Bali
on
Journal of Advances in Tropical Biodiversity and Environmental Sciences 3(1): 17-20, February 2019 (p-ISSN: 2549-6980)
DOI: 10.24843/atbes.v03.i01.p05 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/50706
17
Seagrass (Enhalus acoroides) as an Heavy Metal Bioindicator on Biomonitoring Water Quality in Sanur Beach, Bali
Ni Putu Putri Wijayanti1* and I Nyoman Giri Putra2
Program Study of Aquatic Resource Management1, Program Study of Marine Science2 The Faculty of Marine Science and Fisheries, Udayana University Jimbaran, Bali
*Corresponding author: putri_wijayanti@unud.ac.id
Abstract. Heavy metal contamination is a major threat for marine ecosystem that directly affecting the organisms’ life. Some of marine organisms have an ability to response the heavy metal contamination in water, and could be used as bio-indicator. One of them is seagrass that used in this study to evaluate the water quality in Sanur beach. This study was held in Sanur beach, Denpasar, from July-August 2018. Sampling location determined by purposive sampling method and generated 4 research stations. Sediments and seagrass leaves (Enhalus acoroides) was collected and analyzed in Analytical Laboratory Udayana University using ICPE-9000. Water quality parameters measured in situ (temperature and salinity) and in Medical Laboratory Bali Province (pH and DO). The results showed if the water quality in all research stations were in safety range based on Governor Regulation No. 16 of 2016 about marine water quality for tourism and recreation. The highest content of heavy metal (Pb) in sediment is 16.207 mg kg-1 that possessed by the 1st station, while the lowest is 14.318 mg kg-1 at the 3rd station. The highest Pb content in seagrass leaves at the 2nd station is 5.646 mg kg-1, and the lowest at the 1st station is 4.926 mg kg-1. The 3rd station sediment had the highest Cd content is 2.252 mg kg-1 and the 4th station had the lowest is 2.044 mg kg-1, while for leaves with the highest Cd content was at the 4th station is 0.552 mg kg-1 and the lowest at the 3rd station is 0.458 mg kg-1. The Cu content in sediment was highest at the 1st station is 11.533 mg kg-1 and the lowest at the 3rd station is 8.501 mg kg-1. For leaves’ Cu content, the highest amount of Cu is 3.699 mg kg-1 at the 4th station, while the lowest at the 2nd station with 2.570 mg kg-1.
Keywords: biomonitoring, bioindicator, contamination, heavy metal, seagrass
-
I. INTRODUCTION
Sanur beach is one of the most favorite destination areas for both domestic and international tourist. The massive activity of speedboats crossing the sea affects the quality of Sanur beach’s water. One of those causes is the contamination of heavy metal derived from the residual of speedboat fuel. Those residuals would accumulate in the water body and absorbed by living organisms, that induce serious threat to marine ecosystem, including mangrove, coral, and seagrass [1].
Seagrass is a marine plant that has high capacity to absorb the heavy metal pollution by its interaction with the water body and sediment [1]. The increase of heavy metal content in water body would affect the metabolism of marine organisms and the toxicity would disturb the marine ecosystem [2]. To assess the heavy metal pollution threat, measurement of the heavy metal content should be done for water body and the marine organisms. Some of
marine organisms could be a bioindicator for marine ecosystem by evaluating the accumulation of heavy metal content [3]. One of those organisms is seagrass Enhalus acoroides that used as a bioindicator to assess the water quality in Sanur beach, Denpasar, Bali. Indirectly, that seagrass could be used as a biomonitoring of the heavy metal pollution in Sanur beach.
-
II. RESEARCH METHOD
Descriptive-quantitative method was used to describe event and recent condition of water quality and marine ecosystem in Sanur Beach. Purposive random sampling used to determine the sampling area that composed by 4 research stations, there are (1) the Lembongan cross station, (2) the Nusa Penida cross station, (3) the tourism activity station, and (4) the canoe station at Sanur Beach (Figure 1).
DOI: 10.24843/atbes.v03.i01.p05 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/50706
The seagrass samples selected by its size, varies from 20–25 cm, and the leaves that sampled were the mature leaves that relatively similar among the all 4 research stations. Those leaves then gently washed in clean water to remove the sediments and other contaminant. The leaves then placed in plastic, labelled by its research stations, and stored in cool box. Sediment collected by shovel, placed in plastic and labelled, then also stored in cool box. The leaves and sediments samples then analyzed in Analytical Laboratory of Udayana University to assess the heavy metal content, specific for Pb, Cd, and Cu.
Figure 1. Map research location
The analysis of bio concentration factor based on the heavy metal content in the organisms divided by the heavy metal content in the sediment, using the formula below (Connell dan Miller, 1995):
BCF = Kb∕Cw
Note:
BCF = bio concentration factor
KB = heavy metal concentration in organism CW = heavy metal concentration in sediment
Category:
-
1. Accumulator: if the value of BCF >1. Accumulator is a plant they could absorb the high concentration of heavy metal in its tissue more than in soil (sediment)
-
2. Excluder: if the value of BCF < 1. Excluder is a plant that effectively prevent the accumulation of heavy metal content in its tissue, so the heavy metal content higher in soil than in tissue.
-
3. Indicator: if the value of BCF nearly 1. This category for the plant that tolerate with the heavy metal pollution by synthesize specific compound that bind with heavy metal or by store the heavy metal content in the insensitive parts [4].
18 III. RESULT AND DISCUSSION
Water Quality Parameter
Water quality measurement including temperature, acidity (pH), salinity, and dissolved oxygen (DO). Based on the measurement, the water quality in Sanur beach is still in safety range based on Governor Regulation No.16 of 2016 The result of water quality measurement showed in Table I.
TABLE I
MEASUREMENT OF WATER QUALITY
|
Parameter |
Research Station |
Max Range (Governor Regulation No 16 of 2016) | |||
|
1 |
2 |
3 |
4 | ||
|
Sampling I Temperature |
27 |
27,1 |
27,6 |
27,5 |
26-30 |
|
(°C) Acidity (pH) |
7,50 |
7,00 |
7,00 |
7,00 |
6,5-8,5 |
|
Salinity (‰) |
32,3 |
32,7 |
33,3 |
33 |
Natural |
|
DO (mg/l) |
8,46 |
8,35 |
8,40 |
8,40 |
> 5 |
|
Sampling II Temperature |
29,8 |
29,0 |
29,7 |
29,2 |
26-30 |
|
(°C) Acidity (pH) |
8,27 |
8,08 |
8,00 |
8,49 |
6,5-8,5 |
|
Salinity (‰) |
31,7 |
30 |
30,3 |
31,3 |
Natural |
|
DO (mg/l) |
8,03 |
8,21 |
8,68 |
8,43 |
> 5 |
|
Sampling III Temperature |
26,9 |
26,8 |
26,8 |
26,5 |
26-30 |
|
(°C) Acidity (pH) |
8,35 |
8,22 |
8,21 |
8,21 |
6,5-8,5 |
|
Salinity (‰) |
33 |
33 |
32,7 |
33,7 |
Natural * |
|
DO (mg/l) |
8,13 |
8,18 |
8,01 |
7,81 |
> 5 |
Based on the research, the water temperature in Sanur beach ranged from 27.7 – 28 ºC and in range of seagrass
optimum temperature. Previous studies states that seagrass optimum temperature ranged from 20-36 °C and 28-30 ºC for optimum photosynthesis [5]. Temperature is essential to seagrass metabolism. The change of water temperature would affect the nutrient absorption, photosynthetic activity, respiration, and in general for growth and development of seagrass. At 40°C or more, seagrass would stress and die [6].
The pH in water of Sanur beach is 7.74-8.04 and still in normal range based on Pergub Bali No. 16 Tahun 2016. The optimum pH for seagrass is 7.5-8.5, to maintain the equilibrium of bicarbonate ion for photosynthesize [7].
For salinity, seagrass have different level of salinity, but generally ranged from 10-40 ppt [8] and optimum in 35 ppt. the salinity in this research in 31.9-32.7% and in safety range based on Peraturan Gubenur Bali No. 16 Tahun 2016. Low salinity would be increasing the heavy metal concentration in water body by causing the decrease of chloride [9].
Dissolved oxygen (DO) showed the amount of oxgen in water. The DO measurement in Sanur beach ranged Hasil 8.21-8.36 mg/L. Effendi (2003) states that in water body,
DOI: 10.24843/atbes.v03.i01.p05 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/50706
the DO should be not below 5 mg/L, because it would directly be affecting the aquatic organism. The good water body showed by the oxygen diffusion and light intensity that reach the bottom of the water body. Those things would support the growth and development of seagrass especially the photosynthesis that release high amount of oxygen in water [10].
Measurement of Heavy Metal Content in Seagrass Leaves and Sediment
The highest content of Pb in leaves found in the research station 2 with 5.646 mg/kg and the lowest in research station 1 with 4.926 mg/kg. For sediment, the highest Pb content found in research station 1 with 16.207 mg/kg and the lowest in research station 3 with 14.318 mg/kg. High concentration of Pb caused by the activity of speedboats. Fuel residual and boat paint contain Pb that really possible to contaminate the water body [11]. Pb is an additive that mixed in machine fuel, paint, can, and pesticide [12]. The effect of Pb contamination in seagrass absolutely inhibit the growth of seagrass [13]. depigmentation [14], and affecting particular anatomical responses [15].
TABLE II
HEAVY METAL MEASUREMENT IN LEAVE OF SEAGRASS AND SEDIMENT
|
Sample |
Station 1 |
Station 2 |
Station 3 |
Station 4 |
|
Timbale (Pb) (mg/kg) 2,540 5,972 3,155 |
8,085 | |||
|
Leaves |
6,070 |
6,496 |
6,248 |
5,154 |
|
6,169 |
4,469 |
5,816 |
3,327 | |
|
Average |
4,926 |
5,646 |
5,073 |
5,522 |
|
13,153 |
12,426 |
13,891 |
14,259 | |
|
Sediment |
19,917 |
18,506 |
18,968 |
17,962 |
|
15,551 |
17,107 |
10,095 |
16,083 | |
|
Average |
16,207 |
16,013 |
14,318 |
16,101 |
|
Cadmium (Cd) (mg/kg) 0,119 0,248 0,168 |
0,586 | |||
|
Leaves |
0,699 |
0,753 |
0,800 |
0,796 |
|
0,725 |
0,398 |
0,407 |
0,274 | |
|
Average |
0,514 |
0,466 |
0,458 |
0,552 |
|
1,518 |
1,564 |
1,591 |
1,489 | |
|
Sediment |
3,067 |
3,028 |
2,965 |
2,844 |
|
1,874 |
1,849 |
2,201 |
1,800 | |
|
Average |
2,153 |
2,147 |
2,252 |
2,044 |
|
Cuprum (Cu) (mg/kg) 2,239 3,512 2,078 |
4,735 | |||
|
Leaves |
3,686 |
1,651 |
3,942 |
4,482 |
|
4,152 |
2,546 |
3,722 |
1,879 | |
|
Average |
3,359 |
2,570 |
3,247 |
3,699 |
|
11,581 |
10,263 |
10,792 |
10,156 | |
|
Sediment |
13,913 |
10,291 |
9,917 |
8,512 |
|
9,105 |
6,784 |
4,795 |
6,915 | |
|
Average |
11,533 |
9,113 |
8,501 |
8,528 |
The highest of average Cd content in seagrass leaves was in research station 4 with 0.552 mg/kg and the lowest in research station 3 with 0.456 mg/kg. In sediment, the highest Cd found in research station 3 with 2.252 mg/kg and the lowest in research station 4 with 2.044 mg/kg.
19
The highest of average Cu content in seagrass leaves was in research station 4 with 3.699 mg/kg and the lowest in research station 2 with 2.570 mg/kg. In sediment, the highest content of Cu in research station 1 with 11.533 mg/kg and the lowest in research station 3 with 8.501 mg/kg. Cu is one of essential heavy metal for plant, but only in small amount of it. If Cu were too high, it could be turn toxic and affecting the photosynthetic in plant [14]. In water body, Cu naturally occur as a mineral bleach. Authman (2015) states that Cu pollution caused by the use of pesticide and waste of industrial that dumped in water body [16]. The heavy metal in water body would settles in sediment, then bleach and dispersed until absorbed by living organisms that live in water. Heavy metal in sediment absorbed by root of seagrass along with nutrient absoption [15].
Bio Concentration Factor (BCF) in Seagrass Leaves
The appearance of heavy metal in water body causing the accumulation in water organisms. Accumulation in water organisms affected by the food chain that already absorb the heavy metal elements. The calculation of heavy metal content aimed to know the ability of seagrass to accumulate the heavy metal trough bio concentration factor (BCF). Bio concentration factor derived by ratio of heavy metal content in seagrass leaves to sediment. The result showed in seagrass leaves could accumulate Pb about 0.338, Cd 0.232, and Cu 0.347 as shown in Fig. 2. The calculation of BCF in Sanur beach proved that seagrass (Enhalus acoroides) could accumulate heavy metal, these result supported by the research of Supriyanti et al., (2016) [17].
BIO CONCENTRATION FACTOR
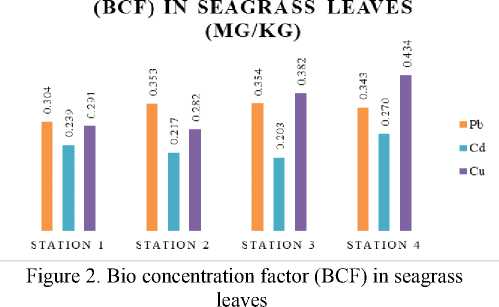
-
IV. CONCLUSION
Seagrass (Enhalus acoroides) in Sanur beach could be used as bioindicator for heavy metal contamination. The water quality and heavy metal analysis in water body of Sanur beach were in safety range based on Governor Regulation No. 16 of 2016
DOI: 10.24843/atbes.v03.i01.p05 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/50706
20 ACKNOWLEDGMENT
The authors tanks to The Rector of Udayana University, and Head of Research and Community Service of Udayana University for funding research. We also very grateful and thanks for the teamwork in this research team.
REFERENCES
-
[1] Tupan, C.I. 2014. Profil logam berat timbal (Pb) di perairan Pulau Ambon dan dampaknya terhadap respons struktur anatomi dan fisiologi lamun Thalassia hemprichii (Ehrenberg) Ascherson. Disertasi, Universitas Brawijaya Malang.
-
[2] Connel DW, Miller GJ. 1995. Kimia dan Ekotoksikologi
Pencemaran. Koestoer Y, penerjemah ; Jakarta : Universitas
Indonesia Press. Terjemahan dari : Pollution Chemistry
Ecotoxicology. 520 hlm
-
[3] Acker, L.A., McMAhan, J.R., and Gawel, J.E. 2005. The effect of heavy metal pollution in aquatic environment on methallothionein production in Mytilus sp. Proceeding.
-
[4] Baker AJM, Walker PL. 1990. Ecophysiology of Metal Uptake by Tolerant Plants: Heavy Metal Tolerance in Plants. Didalam : Shaw, AJ, editor. Heavy Metal Tolerance in Plants: Evolutionary Aspects. Boca Raton : CRC Press. 155–177 hlm
-
[5] Nybakken, J.W. 1992. Biologi laut suatu pendekatan ekologis.
Eidmen, M. et al. (penterjemah). Sukardjo. PT. Gramedia Pustaka Utama. Jakarta. 459hlm.
-
[6] McKenzie, L.J. 2008. Seagrass Educators Handbook. Seagrass
Watch HQ. http://www.seagrasswatch.org. (Diakses tanggal 1 Pebruari 2018).
-
[7] Philips, C.R., and E.G. Menez. 1988. Seagrass. Smith Sonian
Institutions. Press. Washington.
-
[8] Dahuri, R. 2003. Keanekaragaman Hayati Laut: Aset Berkelanjutan Pembangunan Indonesia. PT Gramedia Pustaka, Jakarta. Hal 305.
-
[9] Palar, H. 2004. Pencemaran dan toksikologi logam berat. Rineka Cipta, Jakarta.
-
[10] Effendi, H. 2003. Telaah kualitas air bagi pengelolaan sumberdaya
dan lingkungan perairan . Penerbit Kanisius, Yogyakarta
-
[11] Järup, L. 2003. Hazards of Heavy Metal Contamination.British
Medical Bulletin Vol.68 Hal: 167–182.
-
[12] Sarong, M.A., C. Jihan, Z.A. Muchlisin, N. Fadli, S. Sugianto.
2015. Cadmium, lead and zinc contamination on the oyster Crassostrea gigas muscle harvested from the estuary of Lamnyong River, Banda Aceh City, Indonesia. AACL Bioflux, 8(1): 1–6.
-
[13] Ambo-Rappe, R., D.L. Lajus, M.J. Schreider. 2011. Heavy metal impact on growth and leaf asymmetry of seagrass, Halophila ovalis.
Journal of Environmental Chemistry and Ecotoxicology, 3(6): 149– 159.
-
[14] Sudharsan, S., P. Seedevi, P. Ramasamy, N. Subhapradha, S.
Vairamani, A. Shanmugam. 2012. Heavy metal accumulation in seaweeds and sea grasses along southeast coast of India. Journal of Chemical and Pharmaceutical Research, 4(9): 4240–4244.
-
[15] Tupan, C. I., R. Azrianingsih. 2016. Accumulation and deposition of lead heavy metal in the tissues of roots, rhizomes and leaves of seagrass Thalassia hemprichii (Monocotyledoneae, Hydrocharitaceae). AACL Bioflux, 9(3): 580–589.
-
[16] Authman, M.M. 2015. Use of fish as Bio-indicator of the effects of heavy metals pollution. Journal of Aquaculture Research and Development, 6(4): 1–13.
-
[17] Supriyantini, E., S. Sedjati, Z. Nurfadhli. 2016. Akumulasi logam berat zn (seng) pada lamun Enhalus acoroides dan Thalassia hemprichii di Perairan Pantai Kartini Jepara. Buletin Oseanografi Marina, 5(1): 14–20.
Discussion and feedback