The Expression Of Cd4+ Lymphocytes Of Bali Cattle After Consuming Mixed Mirerals
on
Journal of Advances in Tropical Biodiversity and Environmental Sciences Vol. 1 No. 2, September 2017 (p-ISSN: 2549-6980)
43
The Expression Of Cd4+ Lymphocytes Of Bali Cattle After Consuming Mixed Mirerals
Ni Ketut Suwiti1, Windhu M1 , Ni Luh Watiniasih3, I Nengah Kerta Besung2, and I Nyoman Suartha2
1Departement of Bali Cattle Research Udayana University 2The Faculty of Veteriner Medicine Udayana University 3The Faculty of Mathematics and Basic Science,Udayana University *Corresponding author: nk_suwiti@unud.ac.id
Abstract. Minerals play an important role in activating the lymphoid cells. Mineral deficiency can cause interference prolifrasi in lymphocytes, particularly the expresion of CD4+. Bali cattle are hardly given additional mineral due to cattles are in semi-intensive breeding method. Therefore, this study aims to determine the expression of CD4+ lymphocytes Bali cattle after consuming of mixed mineral. The samples used were 32 male bali cattles reared in the village of Catur, Kintamani, Bangli regency. Samples were divided into 2 groups, those were: 16 individul of bali cattle was given 7.5 g additional of mixed mineral per individual/day, and theother 16 individual as acontrol. The treatmnes were conducted for 3 months. The results showed that administration of 7.5 g mixed mineral per individual/day did not show differences in the expression of CD4+ lymphocyte of bali cattle.
Keywords: bali cattle, mixed minerals, CD4+, immunocytochemical
-
I. INTRODUCTION
Bali cattle (Bos Javanicus) is indigenous beef cattle, well known due to its superior in breed fertility, conception rate, and adaptable to harsh environments. This breed has a great contribution in Indonesian beef industry, as well as in its role to smallholder farming system [1]. They have hardly given an additional food or minerals to the cattle, therefore the cattle do not maximally grown. For example, the increment of weight of male bali cattle was only 0.32 kg/individual/day, and 0.28 kg/ individual/day of female cattle [2].
Mineral deficiency has also known to more susceptibility of bali cattle to diseases. Diseases that can attack bali cattle is an infectious disease, which include: Jembrana desease, Bovine Viral Diarhea (BVD), Bovine Immunodeficiency Virus (BIV), and diseases caused by bacteria, such as Septicaemia Epizootica, Brucellosis and Fascioliasis. In addition to infectious diseases, bali cattle susceptible to non-infectious diseases such as the metabolic disorder caused by nutrient deficiency, such as minerals [2].
Mineral was grouped into macrominerals calsium (Ca) magnesium (Mg), Sodium (Na) , kalium (K) and Phosphor (P) and microminerals, iron (Fe) copper (Cu), zinc (Zn), copper (Co), manganesa (Mn) [3]. Minerals have a special role in ensuring efficient growth, production [4] and immunocompetence in animals [5].
Mineral is a chemical that has important role in animal growth and development. Calcium (Ca) is a mineral
required as for bones and teeth constituents. Potassium (K) and sodium (Na) controls the osmotic balance of extracellular fluid in the body [6]. Phosphorus (P) in the body plays a role in bone mineralization process [7]. Copper (Cu) required for the process of neurotransmitters and Manganese (Mn) for fat metabolism. In addition, Selenium (Se) and zinc (Zn) is functioning as an antioxidant, and serve as enzyme constituent [8], as well as optimizing the function of the immune response [9]. Chlorines (Cl) are important in the transmission of nerve impulses, muscle contraction.
Macrominerals was required to the development of bones and teeth, and it was also found in lipids, proteins, muscle, and tissue [10]. Based on the primarily observation found that the size of bali cattle decreases through time [2], which can be affected by the amount mineral consumed. Furthermore, evidence suggests that herds have increased risks of metritis, mastitis locomotion problems or diarrhea in calves when zinc (Zn) or copper (Cu) status are either marginal or deficient [11]. Dairy cow feeds typically a range of different compounds that possess antioxidant activities, many of which are minerals or are mineral dependent. The key trace elements involved in animal feed are zinc, copper, iron and manganese [8].
Mineral deficiency results in not optimal of the function of immune response, which will affect the susceptibility of animal to infectious diseases. One indicator of impaired immune response, can be expressed through the appearance of lymphocytes that express CD4+ [8].
-
II. RESEARCH METHODS
The samples used were 32 male bali cattle reared in Catur village, Kintamani, the Regency of Bangli, Bali Province. All of bali cattle were deworming and vaccinated with Septicemia epizootica before treatments. The samples were divided into 2 groups that are 16 individual was treated by adding 7.5g mineral/ individual/day in their food and 16 other individuals without mineral addition in their food, as control group. The treatment of mineral addition to cattle food was carried out for 3 months. Blood samples were collected from treated cattle, then lymphocyte were extracted. The expression of CD4+ was observed using the methods of immunocytochemistry stainied in streptavidin-biotin.
Isolation of lymphocytes was conducted by whole blood centrifugation at 3000 rpm for 15 minutes, the white blood cells that form in the form of a white ring between the two fluids, was taken with a Pasteur’s pipette. The white blood cell was taken and added with RPMI in a tube, homogenized, then put in another tube which contains 5ml of ficolhipaque solution, continued by centrifuging in 3000 rpm for 30 minutes. Interface cells was collected and placed in another tube contained 7ml PBS, then centrifuged in 1250 rpm for 15 minutes. Supernatant was collected then washed twice in PBS then centrifuged for 15vminutes at 1250rpm. The pellet that contained lymphocyte cells was collected and added with 500 µl PBS.
Immunohistochemical analysis was done to analyze the expression of CD4+ with the process as follows: 2 x 106 sample of lymphocyte cells containing polylysine was put in an object glass waiting for drying. The sample fixed in acetone for 10 minutes, then washed in PBS 2x5 minutes. The sample added with 3% H2O2, hold for 20 minutes before it washed in PBS for another 2x5 minutes. One percent of bovine serum albumin (BSA) and monoclonal antibody (AbMo) was added following BoCD4+Kit. Sample was incubated for 18 hours, washed twice in PBS, then soaked in goat anti-mouse IgG (Bio-Rad, USA), incubated for 1 hour. Samples were washed 2 times in PBS for 5 minutes each, then added with Streptavidin-HRP (Dako), preserved for 30 minutes then washed again in PBS. Lymphocyte marker T BoCD4+ was visualized by adding diamino 0.01% benzidine (DAB) (SigmaTM, USA). The reaction of the substrate was stopped by washing in flowing tap water for 15 minutes, and dropped with mayer hematoxylin, after 3 minutes washed in bicarbonated water. After sealed with glycerin jelly, the sampel then covered with cover glass and observed under a microscope.
CD4+ lymphocytes expressed in brown colour, checked with 400x magnification in five visual fields clockwise. The calculation of histologal score was by multiplying the percentage of staining intensity. The histologal score = (IKxPK) + (ISxPS) + (ILxPL) + (IN x PN). Note: I = intencity, P = percentage, K = strong positive intensity, S = moderate positive intensity, L= weak positive intensity, N = negative intensity. Percentage:
Score 0: no positive cell, 1: positive cell 1–25%, 2: positive cell 26–50%, 3: positive cell 51–75%, 4: positive cell 76– 100%. Intensity: Score 0: not stained/ negative, 1: weak positive, 2: moderate positive, 3: strong positive.
-
III. RESULTS AND DISCUSSION
The expression of CD4+ lymphocytes of bali cattle with additional mineral of 7.5g/head/day and control, is presented in Figure 1. The expression of CD4+ lymphocytes of bali cattle given mineral of 7.5g/head/day was higher (6.65±0.47) compared to control group (6.47±0.34), but there was no significant difference (P>0.05). The result show that the average expression of CD4+ at the treatment group was higher (6,65±0,47), but not significantly different to the control group (6,47±0,34). This mighy due to the amount of mixed mineral given was unable to maximized the appearance of CD4 expression+. Another factor that can influence is the interactions between mineral elements, which can be either synergistic or antagonistic [12]. A high concentration of antagonic elements may reduce the effectiveness of the biological target element. Antagonistic interaction is often expressed as mutually inhibitory interactions during absorption in the intestine or at the cellular level [13].
In sufficient of mineral given during treatment may result in low expression of CD4+ in bai cattle. Some minerals such as Cu can optimize the function of the immune response, but the absorption of copper requires considerable time to be able to have an effect to the target organ. Absorption occurs in the digestive tract, into the bloodstream and binds in protein albumin. Copper delivered and released to the liver and kidney tissues and binds to proteins to form enzymes, especially the enzyme ceruloplasmin copper, containing 90-94% of the total copper content in the body [14]. The amount of minerals required for the physiological prosesses in animals depend on the state of the animal [6]. For example, it depends on the species, type or race, sex, age, nutritional and health status, as well as seasonal and physiological variations such as those in pregnancy and lactation [15, 16]. Cu was important physiologically during animal gestation and lactation [17]. Trace mineral status, especially selenium (Se) and zinc (Zn), affects neutrophil function in postpartum cows [18].
In this absorption process interaction between copper, molybdenum and sulfate can occur. Sulfites are formed by rumen microbes derived from sulfate or organic sulfur from the feed. Sulphite reacts with molybdate to form thiomolibdat which then binds copper to copper tiomolibdat (CuMoS4), which is not soluble in water, so it can not be absorbed by the intestine. The imbalance ratios between the Cu and Mo are very influential on copper absorption in ruminants, because of the presence of bacteria in the rumen, which able to produce sulfide [19, 20].

Fig. 2. The average expression of CD4+ lymphocytes of bali cattle were given mineral and control
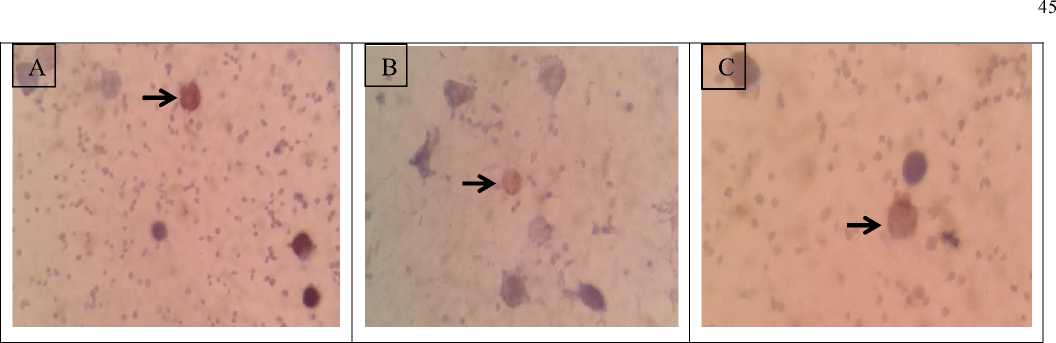
Fig. 1. Lymphocytes of bali cattle (Streptavidin-biotin, 1000X), the expression of CD4+ colored brown. A: strong intensity, B: moderate intensity and C: weak intensity.
The absence of different expression of CD4+ may be due to short of treatment time (3 months), given some of the need longer time to be absorbed, and should be balance with the presence other minerals. The quality of feed is a complex of several interrelated factors evaluated in several ways. An important indicator is the mineral content. Monitoring the mineral content is important in terms of the ecosystem (cycle minerals) but also in terms of quality feed [21 ]. Mineral contents in forages depend on soil, drainage and pH, forage species and varieties, forage maturity, pasture management, forage yield and climate [22]. A natural source of minerals in the diet of farm animals is feed [7]. Utilization minerals from food can be changed to different combinations of feed and representation grasses, legumes and herbs. Forages species vary widely in the range and quantity of trace elements. Fertilizer application may also affect trace elements concentration [23 ].
The absorption of Zn involves the role of transferrin, which is also the material for transportation Fe. When the proportion of iron and zinc is more than 2: 1, the available of transferrin for Zn will be reduced, thus inhibiting the absorption of Zn. On the other hand, high dose on inhibits the absorption of Fe [11 ]. Fe absorption disorders can also be caused by certain compounds that has capability to bind with iron to form unsoluble complex compounds, making it difficult or can not be absorbed in the intestinal wall. A
number of compounds known to inhibit iron absorptions are tannins, phytate and food fibers. The expression of CD4+ which was higher in bali cattle provided additional mineral than controls, demonstrating that the existence of minerals in the body of bali cattle have an impact, as the marketed mineral mix was aimed to increase productivity. Non-significant different of CD4 contents in treated bali cattle and control cannot be explain further due to lack of pre data on mi+neral content in the blood of bali cattle before treatments, but all bali cattle used in this research were in good and healthy state.
BoCD4+ was expressed in bali cattle when they are suffered from jembrana virus diseases [24]. The number of CD4+ increased significantly in people who suffered from HIV/AIDS when they were administered with Zn sulfate. The virus of HIV/AIDS attacks active CD4+ lymphocytes, resulting in a decrease in the number of CD4+ T lymphocytes [25].
-
IV. CONCLUSION
The supplement of 7.5g/head/day has small effect to the expression of CD4+ lymphocytes bali cattle.
ACKNOWLEDGEMENT
This research was funded by Ministry of Higher Education Research Grant trough Udayana University. Thanks to I Nyoman Mantik Astawa, the Head of Patobiology Laboratory, Faculty of Veteriner Udayana University for his help and permission, allowing to conduct part of the research in his Lab.
REFERENCES
-
[1] Dahlanuddin K, Ningsih BS, Poppi DP, Anderson ST, Quigley SP. 2014. Long-term growth of male and female Bali cattle fed Sesbania grandiflora. Animal production science. 54 : 1615-1619
-
[2] Suwiti NK, Wijayanti, Rumbawa, INK Besung. 2012. Bobot badan dan Umur Sapi Bali yang Dijual di Pasar Hewan dalam Hubungannya dengan Produksi Daging. Proc. Seminar Nasional. Pusat Kajian Sapi Bali Unud-Bali. 14 September 2012.
-
[3] Mandal AB, Yadav PS, Kapoor V. 2004. Mineral profile and their retention in lactating cows in relation to soil fodder and feed in Kamrup district of Assam. Indian J Anim. Sci. 71: 421-429.
-
[4] Moeini MM, Kiani A, Karami H, Mikaeili E. 2011. The effect of selenium administration on the selenium, copper, iron and zinc status of pregnant heifers and their newborn calves. J Agri. Sci Tech 13: 53-59
-
[5] Peres JM, Bureau F, Neuville D, Arhan P, Bugle D. 2001. Inhibition of zinc Absorption by Iron Depends on their Ratio. Journal of Trace Elements in Medicine and Bipology 15:237-241
-
[6] Smith D. 2005. A survey of selected heavy metal concentrations in Wisconsin Dairy Feeds. Journal Dairy Science 88: 2911-292225 Asikin A, Bambang W, Soeroso J. 2012. Zinc Sulfate Increases Lymphocyte CD4 Count in Hiv/Aids Patients at ICUID Dr. Soetomo Hospital Surabaya. Folia Medica Indonesiana. Vol. 48 (1) : 17-19.
-
[7] Khan ZI, Ashraf M, Ahmad K, Mustafa I, Danish M. 2007. Evaluation of Micro Minerals Composition of Different Grasses in Ration to Livestock Reqruirements. Pak. J. Bot. 39(3): 719-728.
-
[8] Machado VS, Oikonomou G, Lima SF, Bicalho MLS, Kacar C. 2014. The effect of injectable trace minerals (selenium, copper, zinc, and manganese) on peripheral blood leukocyte activity and serum superoxide dismutase activity of lactating Holstein cows. Vet J 200: 299-304.
-
[9] Paik IK. 2001. Application of Chelated Minerals in Animal Production. Asian-Aust. J. Anim. Sci. 14:191 – 198.
-
[10] Paterson JA and Angle TE. 2005. Trace Mineral Nutrition in Beef Cattle. Presented at the 2005 Nutrition Conference sponsored by Department of Animal Science, UT. Extension and University Professional and Personal Development The University of Tennesse. Pp : 1 -22
-
[11] Noaman V, Rasti M, Ranjbari AR, Shirvani E. 2012. Copper, zinc, and iron concentrations in blood serum and diet of dairy cattle on semi-industrial farms in central Iran. Trop Anim Health Prod 44: 407-411.
-
[12] O’Dell BL. 1997. Mineral-Ion Interaction as Assessed by Bioavailability and Ion Channel Function. In : B. L. O’Dell and R. A. Sunde (Eds.) Handbook of nutritionally essential mineral elements. Pp. 641-659. Henry PR, and Miles RD. 2000. Interactions Among
the Trace Minerals. Ciencia Animal Brasiliera 1(2): 95-100.
-
[13] Inoue Y, Osawa A, Matsui Y, Asai Y, Murakami T. Matsui, Yano H. 2002. Changes of Serum Mineral Concentration in Horses During Exercise. Asian Aust. J. Anim. Sci. 15(4): 531-536.
-
[14] Hemati M, Dashtbin HRF, Salari J. 2013. Absopption an Macromineral Interaction in Broiler Production : An Overview. J of Global Veterineria 11 (1) : 49-54
-
[15] Fantuz F, Ferraro S, Todini L, Mariani P, Piloni R, Salimei E. 2013. Essential trace elements in milk and blood serum of lactating donkeys as affected by lactation stage and dietary supplementation with trace elements. J .Animal. 7 (11): 1893-1899.
-
[16] Yokus and Cakir, 2006. Seasonal and physiological Variations in Serum Chemistry and Mineral Concentrations in Cattle. J. of Biological Trace Element Research. 106: 155-266
-
[17] Qin LQ, Wang XP, Li W, Tong X, Tong WJ. 2009. The minerals and heavy metals in cow’s milk from China and Japan. Journal Health Science 55(2): 300305
-
[18] Petering HG. 1980. Some Observations on the Interaction of Zinc, Copper and Iron Metabolism in Lead and Cadmium Toxicity. Environ. Health Perspect. 25: 141–145.
-
[19] Randhawa CS, Randhawa SS, Sood NK. 2002. Effect of Molybdenum Induced Copper Deficiency on Peripheral Blood Cells and Bone Marrow in Buffalo Calves. Asian Aust. J. Anim. Sci. 15(4): 509 – 515.
-
[20] Rabiee AR, Lean IJ, Stevenson MA, Socha MT. 2010. Effects of feeding organic trace minerals on milk production and reproductive performance in lactating dairy cows: a meta-analysis. J Dairy Sci 93: 4239-4251
-
[21] McDowell LR. 1985. Nutrition of Grazing
Ruminants in Warm Climates. Academic Press, Inc. Orlando, Florida.
-
[22] Meyer AM, Reed JJ, Neville TL, Taylor JB, Hammer CJ, Reynolds LP, Redmer DA, Vonnahme KA, Caton JS. 2010. Effects of Plane of Nutrition and Selenium Supply During Gestation on Ewe and Neonatal Offspring Performance Body Composition and Serum Selenium. J. Anim Sci. 88: 1786-1800
-
[23] Berata IK, and Astawa NM. 2011. Identifikasi Sel-sel Target Virus Penyakit Jembrana dengan Teknik Imunositokimia Ganda. Biota Vol. 16 (2): 236-241.
-
[24] Persaud D, Peierson C, Ruff D, Finci KR, Chadwick JB, Margolic A, Ruff N, Hutton S, Siliciano RF. 2000. A Stable Latent Resrvoir for HIV-1 in Resting CD4+ Lymphocytes in Infected Children. J. Clinical Investig, 105: 995-103.
Discussion and feedback