EXERCISE STIMULATES IL-15 AND REGULATES SKIN METABOLISM AND AGING: A LITERATURE REVIEW
on
Sport and Fitness Journal
E-ISSN: 2654-9182 Volume 11, No.3, Sept 2023: 216-224
EXERCISE STIMULATES IL-15 AND REGULATES SKIN METABOLISM IN AGING: A LITERATURE REVIEW
Diah Kartika1, Nila Wahyuni2
1Biomedical Anti Aging Medicine Magister Program, Faculty of Medicine, Universitas Udayana, Denpasar, Bali, Indonesia.
2Department of Physiology, Faculty of Medicine, Universitas Udayana, Denpasar, Bali Indonesia.
Email: kartikachndraa@gmail.com
ABSTRACT
The role of immunology in improving skin metabolism in aging has emerged, including the involvement of interleukin (IL)-15 induced by exercise in modifying skin response. Our paper will review recent findings on how exercise affects the release of IL-15 and other plausible mechanisms related to skin metabolism in aging. We focused on discussing the benefit of exercise based on how it affects the release of IL-15 on a molecular level and its impact on both skin metabolism and the aging process. We performed literature searching in several search engines using (exercise) AND (interleukin-15) OR (skin aging) to find eligible literature. We reviewed 6 out of 114 articles regarding the topic. Some mechanisms are proposed to contribute to the aging process, including the role of DNA damage, and the immune system. IL-15, an exercise-induced proinflammatory factor, is one of the cytokines recently studied. This cytokine may exert its effect through JAK-STAT and AMPK pathways. Additionally, certain regimens of exercise may provide different changes in the IL-15 levels. Exercise may also provide benefits to the skin through the production of NAD+ and extracellular matrices. Certain exercise regiments failed to show the consistency of IL-15 changes while overall helping to improve skin metabolism in aging. Further studies are required to identify the appropriate exercise prescription to maximize the skin health benefit as well as the other skin aging indicators to understand more about the role of exercise to counter the aging process.
Keywords: aging; exercise; interleukin-15; skin metabolism
INTRODUCTION
Exercise, beyond a lifestyle necessity, has been studied extensively in modern medicine due to its tremendous therapeutic benefits. Current studies have dived deeper into its impact and how its prescription might help alleviate the physiological changes done by aging. Aging itself is an inevitable process and the number of people aged 60 years and above will increase from 1 billion in 2020 to 1.4 billion by 2050. This implied a new set of challenges, which is to maintain adequate health even during a progressive age.1
In the emergence of skin health and anti-aging awareness, current studies have focused on multiple approaches to maximize the therapeutic strategies to counter the pathological condition, including the impact of physical activities. It is known that skin has various protective roles for the body, hence its function as the biggest organ of the human body is important to maintain. Skin is the primary protector against pathogens and environmental hazards.2 Any damage or harm to the skin, including the inevitable aging process that imposes on structural change, may compromise its role. Skin aging is associated with increasing skin diseases, such as fungal infections (14.3% – 64%), dermatitis (1% – 58.7%), xerosis (5.4% – 85.5%), and benign skin tumors (1.7% – 74.5%).3 Therefore, understanding how it might be affected by the aging process, is crucial.
Aging is associated with chronic inflammation which is marked by the high circulation of several cytokines and long exposure to antigens. Interleukin (IL)-15 has been known as the T-cell growth factor which further causes increased clearance of antigens. The increased clearance of antigens prevents chronic
inflammation and leads to the prevention of the aging process. Research has also studied the role of immunology in improving skin metabolism, including the involvement of interleukin (IL)-15 induced by exercise in modifying skin response. However, to our knowledge, there is no paper yet concluding the current findings on how exercise may affect the IL-15 level and other indicators of skin metabolism to counter the aging process. Therefore, our paper aims to evaluate recent studies regarding the topic to provide a better understanding and potential research gap.
METHODS
This was a narrative literature review. Eligibility criteria were generated based on the PICO framework. The problem was aging; the interest was exercise; the comparator was any comparator; and the outcome was interleukin-15 and skin metabolism. Based on the PICO framework, the keywords in this review were (exercise) AND (interleukin-15) OR (skin aging) OR (skin metabolism in aging) to perform literature searching. We found 114 articles and reviewed 6 papers after the selection process. We applied the following inclusion criteria: 1) articles written in English, 2) articles published within 2013-2023; and 3) articles discussing the impact of exercise to the level of IL-15 and skin metabolism in aging. When the articles failed to be accessed and were non-original studies, they were excluded from this study. The online databases were PubMed, EMBASE, CENTRAL, and Google Scholar. We included studies comparing exercise to interleukin-15 and skin metabolism which were published in English. The findings were narratively elaborated.
RESULTS
We include six eligible articles in this review. The summary of findings can be seen in Table 1.
Table 1. Studies that evaluate the impact of exercise on the IL-15 level and skin aging indicators
|
Author |
Sample |
Intervention |
Result |
|
Kapilevich et al.4 |
Men aged 18-23 years old divided into 4 groups: weightlifting group (WG), track and field group (TFG), control group 1, control group 2 (CG1/2). WG consisted of 10 elite strength-trained athletes; TFG consisted of 10 elite endurance-trained athletes, and control groups consisted of 10 healthy untrained volunteers each |
|
IL-15 level Static load (pg/ml) Baseline WG: 114.36 ± 5.92 CG1: 38.96 ± 2.52 After Intervention WG: 168.55 ± 7.64 CG1: 42.31 ± 1.75 Dynamic load Endurance training did not increase/decrease the IL-15 in the TFG and CG2 |
|
Shu et al.5 |
61 healthy, sedentary Japanese women (41-59 years old) in Japan assigned to two groups of aerobic training (AT) and resistance training (RT) |
|
AT and RT groups showed increased expression of collagen-encoding genes, hyaluronan synthase, and proteoglycans. Both groups also showed increased IL-15 and myonectin levels. |
dos Santos, Lira, and Antues7
Minuzzi et al.8
and chest press with
Oizumi, Sugimoto, and Aibara6
|
progressively increasing |
RT group specifically showed | |
|
30-64 years old |
loads - The intervention group |
significant increase of proteoglycan-related genes such as biglycan (BGN) and chondroitin sulfate synthase (CHSY1) Intervention group showed |
|
participants with no |
was asked to exercise at |
higher stratum corneum |
|
history of skin diseases |
least 600 Mets/week for 8 |
hydration compared to the |
|
and no history of |
weeks |
control group (p=0.083, |
|
exercising habit 20 non-obese and |
Exercises were performed |
η2=0.10) HIIT |
|
physically active men |
for three times a week |
Day 1 |
|
divided into two |
nonconsecutively |
Pre-exercise: ρ 0,222, p = |
|
intervention groups: high- |
- The HIIT group was |
0,538 |
|
intensity intermittent |
asked to run at 100% |
Post-exercise: ρ 0,232, p = |
|
training (HIIT) and |
velocity correspondent to |
0,519 |
|
moderate-intensity |
maximal aerobic speed |
60 min post-exercise: ρ 0,170, |
|
continuous training |
(MAS) for 1 minute, |
p = 0,638 |
|
(MICT) |
followed by short passive |
After 5 weeks |
|
14 Wistar Rats categorized |
recovery - The MICT group was asked to run at 70% velocity correspondent to the MAS until completing the 5 km The subjects were |
Pre-exercise: ρ -0153, 0 = 0,672 Post-exercise: ρ 0,372, p = 0,290 60 min post-exercise: ρ 0,031, p = 0,933 MICT Day 1 Pre-exercise: ρ 0,023, - = 0,947 Post-exercise: ρ 0,019, p = 0,959 60 min post-exercise: ρ = -0,183, p = 0,612 After 5 weeks Pre-exercise: ρ -0,174, p = 0,630 Post-exercise: ρ -0,122, p – 0,736 60 min post-exercise: ρ = -0,183, p = 0,613 Exercise was found to |
|
into three groups: young |
administered for short- |
increase pJAK1 |
|
sedentary, old sedentary, |
term physical exercise |
phosphorylation compared to |
|
and old exercise |
using a treadmill for 5 days |
aging (P<0,05) but failed to |
increase on JAK2 at
|
Tyr1007/1008 (p>0,05) | |
|
Hingorjo et al.9 |
133 medical students (aged Cardiorespiratory fitness IL-15 level (pg/mL) 17-24 years old) divided measured by Queen’s Group A (p<0,001) into two groups: A (BMI College Step Test Baseline: 4.04 ± 1.60 <23,0 kg/m2) and B (BMI Post-intervention: 9.75 ± 4.23 ≥ 23,0 kg/m2) |
Group B (p<0,001)
Baseline: 3.14 ± 1.44
Post-intervention: 7.18 ± 2.93
DISCUSSION
The aging process results from cumulative damage on the cellular level and alterations of the genetic program. Additionally, our cells are undergoing replicative senescence, a state where the amount of cell divisions is limited until it arrests irreversibly. During its process, one might expect gradual degeneration of the epidermis and dermis. Aging itself is a complex process, amounting from both intrinsic (e.g., genes and hormones) and extrinsic factors (e.g. ultraviolet radiation). As a result, decreased energy metabolism increased mitochondrial oxidative stress, and noticeable deletions of mitochondrial DNA (mtDNA) are all present. Some mechanisms are proposed to contribute to the aging process, including the role of telomeres, DNA damage, and the immune system.10
Telomere is the terminal part of chromosomes in eukaryotic cells and the presence of oxidative stress may modify it. Although its role is to defend cells against degradation, in reality, findings found that telomeres experience shortening that results in the failure of DNA polymerase to replicate the final base pairs of a chromosome. Once it reaches the critical threshold, the cells will experience apoptosis or proliferative senescence through the p53 signaling.10
Any damage to DNA may manifest in skin aging. In many rare occasions where premature aging is found, specific DNA mutations will result in different clinical manifestations of the aging. For instance, the mutation in lamina A, a crucial protein to maintain the organization of chromatin, results in progeria. Another example could be seen in Cockayne syndrome where mutation of helicases exists. Yet, it is universally accepted that across their lifespan, human DNA will have damage accumulation even in the absence of mutation. The role of epigenetics that involves methylation of the DNA still might happen. Some of the common factors susceptible to damage are insulin growth factor and growth hormone.10
Lastly, the involvement of immune system degeneration also contributes to aging and vice versa. The skin at its core is a defensive organ against infections, making it one of the crucial parts of the immune system. Within its tissue lay reticular cells, lymphoid cells, and lymphoid organs and research claimed the amount of Langerhans cells decreases with age. Additionally, since older age contributes to decreased microvascular compliance, the homeostasis of residents' immune cells and pro-inflammatory factors are likely to be affected.11 Other mechanisms that may contribute to the aging include progressive accumulation of reactive oxygen species by age. Studies found that ROS might leak into the cytoplasm due to the aging process and eventually, its overbearing impact leads to immune senescence marked by declining innate immunity to impaired adaptive immune response.10
Interleukin (IL-15), a 14-15 kDA member of the gamma chain cytokine family that is closely correlated with IL-2, is a pro-inflammatory substance that was first known as a T cell
growth factor. Its role also expands to specifically the activation of CD8+ T cells, the development of tissue memory phenotype of CD103+ CD28+ CD8+ T cells, maintenance of naive and memory CD4+ T cells homeostasis, and proliferation of cytotoxic T-cells (CTLs), natural killer (NK) and NKT-like (CD56+CD3+) cells. IL-15 conducts its signal through a heterotrimeric receptor, consisting of IL-15Rα (CD215), IL-2/IL-15Rβ (CD122) and the common γ chain (γc, CD132) that shares receptors with IL-4, IL-7, IL-9, and IL-21. The distinctive feature of IL-15 is its ability to stimulate IL-2/IL-15Rβ and γc-expressing neighboring cells through a mechanism called trans presentation (TP). This finding was initially prompted when the proliferation of T cells induced by IL-15 was not dependent on the membrane expression of IL-15Rα but this receptor aids IL-15 transport through the cytoplasm from the endoplasmic reticulum to form IL-15/IL-15Rα complex on the cell surface, hence compensate for its effect despite not being produced in extensive amount.12 The trans presentation allows direct contact of the presenting cells with the responding cell expressing the IL-2/15Rβγc heterodimer. Afterward, the newly formed complex might be internalized by the responding cells and induce activation of signaling by IL-2/15Rβ and γc (13). Afterward, the signaling process is initiated. It is stipulated that the release of IL-15 is affecting four major pathways: JAK-STAT, PI3K-AKT/mTOR, Ras-RAF/MAPK, and AMPK.
Activation of the JAK-STAT pathway is necessary for the differentiation of the T helper (Th) cells. The next two pathways, PI3K-AKT/mTOR and Ras-RAF/MAPK are responsible for T cell homeostasis involving survival, proliferation, and maintenance, with emphasis on the former pathway contributing to the balance of Treg cells and other CD4+ cells.13 It is known that age affects the amount and function of T cells significantly leading to a phenomenon called immunosenescence and therefore, any modulation to amplify the T cells might be beneficial to prevent the aging impact.14
The theory of how IL-15 correlated with the AMP-activated protein kinase (AMPK) pathway was proven by a study that found reduced serum IL-15 in mice lacking muscle AMPK. This pathway is activated once increasing levels of AMP and ADP are found after physiological stresses are given. AMPK itself is a heterotrimeric substance composed of important subunits: α, β, and γ. Consequently, this pathway triggers cellular metabolism and may alter autophagy and aging.15,16
Interleukin-15 has been found to possess anabolic effects in cells and its production is highly affected by activities made on the skeletal muscles. Nielsen et al. demonstrated that this cytokine is predominantly expressed by type II skeletal muscle fibers. Fundamentally, there are two common types of muscle fibers, mainly type I or slow-twitch fibers and type II or fast oxidative glycolytic (FOG) fibers. The slow-twitch fibers are most commonly found in the soleus muscle, where it has slower twitch speeds but is relatively fatigue resistant compared to the type II fibers, such as the triceps. Certain muscles are dominated with one type of fibers than the other, hence certain exercises focusing on the respective muscle would be responsible for eliciting different responses. One study by Nielsen et al. found that resistant exercises elicited higher IL-15 mRNA levels twofold higher in the triceps compared to soleus muscles.17 In the long run, the process promotes overall health as IL-15 helps to augment the glucose uptake through increased transcription and the translocation of GLUT4 membrane through the JAK3/STAT3 signaling.18
Through the STAT3 pathway, the exercise-induced IL-15 was studied to have a beneficial impact on the impaired wound healing process, one that is commonly found in the aged population. This cytokine reduces biomarkers responsible for growth suspension and stimulates keratinocyte and fibroblast growth. This process is proven by a study by Wong et al., where low-dose injection of IL-15 in vivo improves wound closure, and mitochondrial activity, and decreases the senescence
of cells. This finding was linear with previous research that IL-15/IL-15Rα expression increased both in healing and non-healing chronic wounds respectively. The cytokine was claimed to promote the proliferation of human adult low calcium temperature (HaCaT) keratinocytes, suggestively through the AKT and ERK pathway by exerting anti-apoptosis properties on epidermal keratinocytes.2,19
However, while IL-15 is highly expressed in the skeletal muscle, in which manner exercise produces IL-15 is still debatable. A recent study by Santos, Lira, and Antunes found that high-intensity intermittent training (HIIT), showed neither acute nor chronic significant changes in IL-15 levels, responding to five weeks of HIIT. This proves that muscle damage in healthy young men does not release the IL-15 cytokine.7 Similarly, this level of the cytokine and JAK/STAT pathway in aged rats is not changed after short-term physical activities.8 Yet, Hingorjo et al. and Kurz et al. reported the opposite findings, on which endurance and aerobic exercises, even with short duration, may support the changes of IL-15 to exert its therapeutic effect.9,20 Further studies regarding the prescription of exercises to affect the changes in the cytokine level must be conducted to provide maximum benefit.
Besides demonstrating its impact through the release of IL-15 cytokine, exercise may insinuate more active metabolism and anti-aging through several processes. One of the most commonly discussed metabolites in the correlation between exercise and skin metabolism is the nicotinamide adenine dinucleotide (NADH). NAD+ holds important cellular functions at the biomolecular level by repairing DNA, remodeling chromatin, and controlling cellular senescence and immune cell functions.21,22 One study found that exercises to exhaustion may change NADH levels at rest, proving that physical activities may exert an anti-aging effect.23,24
Exercise may also help to rejuvenate skin aging. Research by Oizumi, Sugimoto, and Aibara supports that attempts of regular exercises aid to improve skin moisturizing function marked by increased hydration.6 Additionally, one study has demonstrated that both aerobic and resistance training improves skin elasticity and upper dermal structure, with additional skin thickness improvement on resistance training. This process is marked by increased extracellular matrices and reduced inflammatory factors. In resistance training, the skin expresses lower circulating levels of CCL28, N,N-dimethylglycine, and CXCL4, followed by an increase in local biglycan (BGN) expression.5 It is known that the degradation of ECM may accelerate skin aging and increase skin stiffness, which the process is mediated by the glycation process. Glycation itself is a non-enzymatic reaction of free reducing sugars with free amino protein groups, DNA, and lipids which happens spontaneously. The glycation itself produces advanced glycation end products (AGEs) and they are mostly reduced in subjects who undergo regular aerobic exercise such as treadmill training.25,26
Other mechanisms where exercise modulates the anti-aging process can be seen through increased collagen synthesis. The amount of collagen usually decreases with age due to the impact of both internal and external (photo-aging) factors. Collagen plays an important role in maintaining the skin's integrity, therefore causing apparent wrinkles, reduced moistness, elasticity, and hydration.27 Through the AMPK pathway, exercises may stimulate collagen production and consequently contribute to anti-aging. An in vivo study seeks the benefit of endurance training and it was found to increase the collagen content, together with extensive remodeling that revitalizes the thickness of stratum spinosum.16 The AMP-AMPK regulation towards the NLRP3 inflammasome contributes to aging which is mostly seen in systemic age-related diseases. In correlation with how it maintains skin health, AMPK is found to suppress the nuclear factor kappa B (NF-kB) activation which in return regulates the innate immunity and inflammation process.
Additionally, there is decreasing AMPKα1 and AMPKα2 expression with advanced age and the study confirmed that in AMPKa2 KO mice, the samples show accelerated skin aging.28 The collagen synthesis is also supported by the mediating effect of previously discussed IL-15 which in return also increase the fibroblast and dermal collagen productions.4 The diagram showing the role of exercise in IL-15 level and overall skin metabolism in aging can be seen in Figure 1.
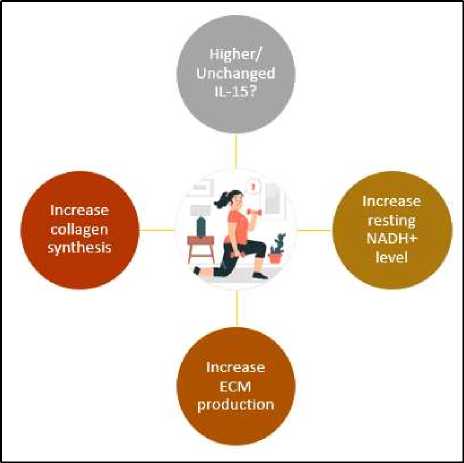
Figure 1. Role of exercise in IL-15 level and overall skin metabolism in aging
CONCLUSION
Exercise, beyond a lifestyle necessity, has been studied extensively in modern medicine due to its tremendous therapeutic benefits including skin aging. Exercise has shown potential benefits to counter the aging process through several mechanisms, namely the increase of IL-15 levels, increased extracellular matrix production, and NADH production. However, further precaution has to be made in concluding the study result as certain exercise regiments failed to show consistency in the IL-15 level changes before and after exercise. Additionally, we have not found further how IL-15 may correlate directly to the gross clinical appearance of the anti-aging process in the skin. Further studies are required to identify the appropriate exercise prescription to maximize the skin health benefit as well as the other parameters of skin aging to better understand the role of exercise in this process of countering aging.
CONFLICT OF INTEREST
There is no conflict of interest related to the materials or methods used in this study.
FUNDING
This article received no specific funding from any funding agency in the public, commercial, or not-for-profit sectors.
AUTHOR CONTRIBUTION
The authors took part in the manuscript, contribute to data collection, and participated in writing the manuscript and all agree to accept equal responsibility for the accuracy of the content of this article.
REFERENCES:
-
1. World Health Organization. Ageing and health [Internet]. World Health Organizations.
[cited 2023 Apr 26]. Available from: https://www.who.int/news-room/fact-
sheets/detail/ageing-and-health
-
2. Wong W, Crane ED, Kuo Y, Kim A, Crane JD. The exercise cytokine interleukin-15 rescues slow wound healing in aged mice. J Biol Chem. 2019; 294(52): 20024 – 38.
-
3. Hahnel E, Lichterfeld A, Blume-Peytavi U, Kottner J. The epidemiology of skin conditions in the aged: a systematic review. J Tissue Viability. 2017; 26(1): 20 – 8.
-
4. Kapilevich L V, Zakharova AN, Kabachkova A V, Kironenko TA, Orlov SN. Dynamic and static exercises differentially affect plasma cytokine content in elite endurance-and strength-trained athletes and untrained volunteers. Front Physiol. 2017; 8: 35.
-
5. Nishikori S, Yasuda J, Murata K, Takegaki J, Harada Y, Shirai Y, et al. Resistance training rejuvenates aging skin by reducing circulating inflammatory factors and enhancing dermal extracellular matrices. Scientifi Rep. 2023; 13: 10214.
-
6. Oizumi R, Sugimoto Y, Aibara H. Effects of regular exercise on skin moisturizing function in adults. Dermatology Reports. 2023.
-
7. dos Santos T, Lira FS, Antunes BM. Interleukin-15 and creatine kinase response to high-intensity intermittent exercise training. Sport Sci Health. 2020; 16: 479 – 84.
-
8. Minuzzi LG, da Conceição LR, Muñoz VR, Vieira RFL, Gaspar RC, da Silva ASR, et al.
Effects of short-term physical training on the interleukin-15 signalling pathway and glucose tolerance in aged rats. Cytokine. 2021; 137: 155306.
-
9. Hingorjo MR, Zehra S, Saleem S, Qureshi MA. Serum Interleukin-15 and its relationship with adiposity Indices before and after short-term endurance exercise. Pakistan J Med Sci. 2018; 34(5): 1125.
-
10. Kerns ML, Chien AL, Kang S. Fitzpatrick’s Dermatology in General Medicine, 9e.
-
11. Russell-Goldman E, Murphy GF. The pathobiology of skin aging: new insights into an old dilemma. Am J Pathol. 2020; 190(7): 1356–69.
-
12. Luo Z, He Z, Qin H, Chen Y, Qi B, Lin J, et al. Exercise-induced IL-15 acted as a positive prognostic implication and tumor-suppressed role in pan-cancer. Front Pharmacol. 2022; 13: 1053137.
-
13. Kenesei Á, Volkó J, Szalóki N, Mocsár G, Jambrovics K, Balajthy Z, et al. IL-15 transpresentation is an autonomous, antigen-independent process. J Immunol. 2021; 207(10): 2489–500.
-
14. Li M, Yao D, Zeng X, Kasakovski D, Zhang Y, Chen S, et al. Age related human T cell subset evolution and senescence. Immun Ageing. 2019; 16: 1 – 7.
-
15. Salminen A, Kaarniranta K, Kauppinen A. Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res Rev. 2016; 28: 15 – 26.
-
16. Crane JD, MacNeil LG, Lally JS, Ford RJ, Bujak AL, Brar IK, et al. Exercise‐ stimulated interleukin‐ 15 is controlled by AMPK and regulates skin metabolism and aging. Aging Cell. 2015; 14(4): 625 – 34.
-
17. Plotkin DL, Roberts MD, Haun CT, Schoenfeld BJ. Muscle fiber type transitions with exercise training: Shifting perspectives. Sports. 2021; 9(9): 127.
-
18. Gonzalez-Gil AM, Elizondo-Montemayor L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: a review. Nutrients. 2020; 12(6):1899.
-
19. Jones AM, Griffiths JL, Sanders AJ, Owen S, Ruge F, Harding KG, et al. The clinical significance and impact of interleukin 15 on keratinocyte cell growth and migration. Int J Mol Med. 2016; 38(3): 679 – 86.
-
20. Kurz E, Hirsch CA, Dalton T, Shadaloey SA, Khodadadi-Jamayran A, Miller G, et al. Exercise-induced engagement of the IL-15/IL-15Rα axis promotes anti-tumor immunity in pancreatic cancer. Cancer Cell. 2022; 40(7): 720 – 37.
-
21. Covarrubias AJ, Perrone R, Grozio A, Verdin E. NAD+ metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021; 22(2): 119 – 41.
-
22. Bugaj O, Zieliński J, Kusy K, Kantanista A, Wieliński D, Guzik P. The effect of exercise on the skin content of the reduced form of NAD and its response to transient ischemia and reperfusion in highly trained athletes. Front Physiol. 2019; 10: 600.
-
23. de Guia RM, Agerholm M, Nielsen TS, Consitt LA, Søgaard D, Helge JW, et al. Aerobic and resistance exercise training reverses age ‐ dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol Rep. 2019; 7(12): e14139.
-
24. Poljsak B, Milisav I. NAD+ as the link between oxidative stress, inflammation, caloric restriction, exercise, DNA repair, longevity, and health span. Rejuvenation Res. 2016; 19(5): 406 – 13.
-
25. Kim C-S, Park S, Kim J. The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise. J Exerc Nutr Biochem. 2017; 21(3): 55.
-
26. Karas A, Holmannova D, Borsky P, Fiala Z, Andrys C, Hamakova K, et al. Significantly Altered Serum Levels of NAD, AGE, RAGE, CRP, and Elastin as Potential Biomarkers of Psoriasis and Aging—A Case-Control Study. Biomedicines. 2022; 10(5):1133.
-
27. Al-Atif H. Collagen supplements for aging and wrinkles: a paradigm shift in the fields of
dermatology and cosmetics. Dermatol Pract Concept. 2022; 12(1).
-
28. Cordero MD, Williams MR, Ryffel B. AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends Endocrinol Metab. 2018; 29(1): 8 – 17.
224
Discussion and feedback