EVALUATION OF MORPHOLOGICAL CHARACTERS AND ASSESMENT OF DNA CONTENT USING FLOWCYTOMETRY ANALYSIS IN REGENERANTS DWARF NAPIERGRASS FROM EMBRYOGENIC CALLI
on
pastura Vol. 3 No. 1 : 35 - 37
ISSN : 2088-818X
EVALUATION OF MORPHOLOGICAL CHARACTERS AND ASSESMENT OF DNA CONTENT USING FLOWCYTOMETRY ANALYSIS IN REGENERANTS DWARF NAPIERGRASS FROM EMBRYOGENIC CALLI
Nafiatul Umami
Lab. Hijauan Makanan Ternak dan Pastura, Fakultas Peternakan, Universitas Gadjah Mada, Jl. Fauna No 3 Kampus Bulaksumur UGM, Yogyakarta e-mail: nafiatul.umami@ugm.ac.id
ABSTRACT
Callus is an excellent source for in vitro plant regeneration, but plants regenerated from callus sometimes show phenotypic and genotypic variation from the initial plants. In this study, there was no variations between regenerated plants and dwarf napiergrass as control. Research result on six morphological characters did not show differences between regenerated plants and control plants. There were not either significant differences of DNA content between control and regenerated plants. The method established by this research was a stable and efficient method that can be applied for supplying transformation materials using callus.
Key words: Callus, morphological characteristic, dwarf napiergrass
INTRODUCTION
The major goal of plant breeding is to improve existing cultivars and to develop new or elite bud by in vitro culture. The genetic variability induced by in vitro culture has been exploited to serve such breeding purpose due to the fact that the plants obtained from in vitro culture sometimes show phenotypic and genotypic variations from initial plant. This phenomenon is called “somaclonal variation”, which refers to the variation arising in cell culture, regenerated plants and their progenies (Larkin and Scowcort 1981).
The genetic basis of somaclonal variation is not fully understood yet. However, it is postulated that the events such as large scale deletions and gross changes in chromosome structure/ number and directed and undirected point mutations, transposon activation and epigenetic changes such as DNA methylation, hystone acetylation and chromatin remodeling, contribution to the in vitro variations.
A wide range of altered phenotypic expression in regenerated plants can be found, such as chlorophyll deficiency, dwarfs, seed characteristics, reproductive structures and necrotic leaves (Phillips et al. 1994). However in contrast, somaclonal variants are undesired when the tissue culture protocols applied for developing transgenic plants since the occurence of somaclonal variants will complicate the evaluation of the effects of inserted gene (s), thus constitutes a major problems (Lutts et al. 2001).The resulting genetic variability can be consequential; therefore it is imperative to check for genome stability of in vitro regenerated plants. The stability of plant genomes can be affected by the in vitro conditions to which the plants are subjected during the propagation process. With each plant species responds differently. Chromosomal/ genome size changes occuring during the in vitro propagation have been reported in several gramineae. Flow cytometry has been described as one of the most reliable technique to
estimate nuclear DNA contents in plants. In comparison with other methods, as feulgen microdensitometry and chromosome counting, flowcytometry provides unsurpassed easy, speed and accuracy (Dolezel and Bartos 2005). In recent study, this technique has been succesfully applied in the analysis of genom size of brachiaria (Ishigaki et al.2010).
The present report, we reported an evaluation of morphological characters from plant regeneration through inducing somatic embryogenesis from apical meristem of shoot-tillers of regenerated plant and initiated plant and assess DNA content stability of its in vitro regenerated plant compared to initiated native plants using FCM analysis
MATERIALS AND METHODS
Regenerated plant from embryogenic calli culture
Regenerated plants from somatic embryogenesis were produced using established method of embryogenic calli induction, grown well with the some condition in the green house. The aclimated somatic plantlets were maintained in green house for one year. After this preliminary phase aiming at describing and asesing the egenerants population, all somatic embryogenesis regenerated plants transfer to the field for examine the morphological trait of regenerated plants.
On all regenerated plant from embryogenic calli, and respective control plants normal management practices including fertilizer applications were followed during the cultivation in the field. Initiated plant as control origin from stem nod cut of tiller from the field.
Morphological analysis
Rooted tillers of the dwarf napiergrass were transplanted into Wagner’s pot (size 1/2000a; diameter 25 cm depth 30 cm) (1 plant pot–1) filled with commercial heated soil. Each plant was planted at 50 cm x50
cm of spacing. 9 gr of N, P2O5 and K2O per pot of chemical compound fertilizer were applied as dressing in transplanting time. In the greenhouse, plants were gave water at 2–3 d intervals for maintaining the water availability by direct watering to the pots. Field location shows in Figure 1.
|
P 1-3 |
RP 4-2 |
RP 7-3 |
|
RP 3-1 |
RP 6-2 |
RP 1.1 |
|
Cont 1-1 |
RP 4-1 |
Cont 3-3 |
|
RP 6-1 |
Cont 1-3 |
RP 2-3 |
|
Cont 2-3 |
RP 2-1 |
Cont 2-1 |
|
RP 2-3 |
RP 3-3 |
Cont 1-2 |
|
Cont 3-2 |
RP 6-3 |
RP 7-2 |
|
RP 5-1 |
Cont 3-1 |
RP 3-2 |
|
RP 5-3 |
RP 7-1 |
Cont 5-2 |
|
RP 7-2 |
RP 4-3 |
Cont 2-2 |
Figure1. Field design for morphological traits evaluation of regenerated plant (RP) from embryogenic calli and control dwarf napiergrass (Cont).
All observations and measurements (plant heights, internode distance, main stem diameter, number of internode, flag leave blade length, flag leave blade width, panicle length, spikelet number in a panicle and fertile tiller number were done in the second year of the study.
Estimation genome size stability using flow cytometry analysis
Plant materials:
Regenerated plants were analized genome size stability using flow cytometry analysis. Oryza sativa cv Nipponbare (2C=0.91 pg Uozu et al. (1997)) was used as internal references standard. Oryza sativa seedlings were grown in a greenhouse. Native plant dwarf napier grass were collected from Kibana Field, University of Miyazaki and from in vitro propagated planted initiated from embryogenic callus of dwarf napiergrass. The cultures were transferred every 14 days to fresh medium and regenerated shoots were used for subsequent subculturing for shoot multiplication and were maintained in culture for more than 12 months prior to this study.The regenerants were compared to the control plants for six morphological characteristics. The measurements were taken for a) plant length; b) plant height; c) leaf blade length and d) leaf blade width taken from trifoliate leaf of the longest stem; e) number of tiller and f) yield. Each measurement except the yield, was repeated ten times. The mean of the ten measurements was used to define the six morphological characteristics.
Flow cytometry analysis
Flow cytometry was conducted to estimate genome size in this study according to Galbraith et al. (1983) with
few modification. Approximately 0.5 cm2 of young leaves of Oryza sativa cv Nipponbare and 1.0 cm2 of young leaves of native, plant regenerated from embryogenic callus or multiple-shoot clumps of dwarf napiergrass were excised and placed in 90 mm petridishes. The excisates were soaked with 1.0 ml of an extraction buffer containing 50 mM Tris HCl, 0.5% polyninylpyrrolidione, 0.01 % triton-X and 0.63 % sodium sulfite for 5 min and chopped with a razor blade. The suspension including nuclei was filtered through a 50 µm nylon. To stain nuclei completely, 50 µl propodium iodide (PI) and 50 µg ml–1 RNase was added and the sustension was kept at room temperature for at least 5 min.For estimation of DNA content, the FCM was performed using Beckman Cell Lab QuantaTM SC Flowcytometer machine (Beckman Coulter, Inc., Tokyo, Japan) following the method of Ishigaki et al.(2010). The analysis was replicated three times for each sample. DNA content of dwarf napiergrass and regenerated plant were estimated by comparing the fluorescence intensities of the samples derived from the cultivars to that of samples from Oryza sativa cv Nipponbare.
RESULTS AND DISCUSSION
Analysis of morphological characteristics to assess the somaclonal variation in regenerated plants from embryogenic calli shows in Figure 2.

Figure 2. Morphological characteristics and dry matter yield of regenerants from embryogenic calli of dwarf napiergrass and control plants (native dwarf napiergrass) cutting 60 days after transplanting. : control plants and :regenerated plants
Plant height of the regenerants ranged from 110 to 149.3 cm, compared with 128.6 cm in average for the control plants. Plant length of the regenerants varied from 112.1 to 151.5 cm, whereas those of the control plants 134.04 cm in average. Leaf blade length of the regenerants ranged from 52.9 to 80.2 cm compared with 77.4 cm for the control plants. Leaf blade width varied from 2.0 to 3.8 cm compared with 2.9 cm for the control plants. Number of tillers of the regenerants
varied from 12 to 25, compared with 21.6 for the control plants. Fresh yields of the regenerants varied from 449 to 972 g/pot compared with 731.6 g/pot for the control plants. Statistical analysis shows no significant different in morphological characters between regenerants and control plants.
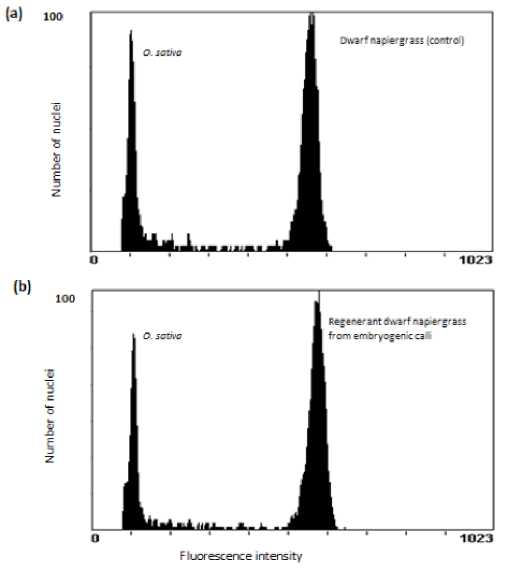
Figure 3. Fluorescent intensity measures of dwarf napiergrass (Pen-nisetum purpureum Schum) and Oryza sativa cv Nipponbare by flow cytometry. (a) Peak 1 corresponds to O. sativa cv Nipponbare, peak 2 corresponds to the control plant (native dwarf napiergrass). (b) Peak 1 corresponds to O. sativa cv. Nipponbare, peak 2 corresponds to regenerant plant from embryogenic calli of dwarf napiergrass.
From FCM analysis, the difference between mean peak position of standar plant prepared in different time, by co-chooping with regenerated plant and control dwarf napiergrass was not significant. This implies that either addition of sample into isolation buffer was able to give respon and do not inhibit PI staining of napiergrass DNA.
The 2C nuclear DNA content of napiergrass was estimated to be 4.72±0.33 pg 2C–1there was no significant difference (P<0.05) in nuclear DNA content between control dwarf napiergrass and in vitro plant regenerated (4.69±0.28 pg 2C–1). This implies that the genome size of regenerated plants produced in vitro remained stable even after long-term culture (>1 years) with repeated sub culturing every 2 weeks. From this study it is inferred that regenerated plant of dwarf napiergrass produced in vitro using this methods maintains the same genom size.
The mean of plant height of regenerated plants and control plants was not significant different, neither was that of the five other observed characters. This means that using this method for EC induction does not promote phenotypic variation. In another research on
Paspalum notatum (Akashi et al. 1992) reported that variations existed in embryogenic calli induction using 2,4-D and BAP, whereas Gondoet al. 2007 andIshigakiet al. 2009, in line with the result of this research, reported the absence of phenotypic variations on the regenerated plants.
FCM analysis was conducted to test the DNA content. This analysis was chosen because it was relatively inexpensive, easy and did not require plentiful samples. The result showed that the DNA content of control plant and regenerated plant were similar. Thus, there were no differences nor somaclonal variation between regenerated and control plants.
This study points out that the establishment method on embryogenic calli induction of dwarf napiergrass, proliferation and regeneration resulted from this research can be applied for producing embryogenic calli as transformation materials because the regenerated plants are stable and similar wih the control plants.
REFERENCES
Akashi R, Adachi T (1992) Somatic embryogenesis and plant regeneration from cultured immature inflorescences of apomictic dallisgrass (Paspalum dilatatum Poir). Plant Sci 82: 213–218.
Gondo T, Matsumoto J, Yamakawa K, Tsuruta SI, Ebina M, Akashi R (2007) Somatic embryogenesis and multipleshoot formation from seed-derived shoot apical meristems of rhodes grass (Chloris gayana Kunth). Grassl Sci 53:138–142.
Dolezel J, Bartos J. 2005. Plant DNA flow cytometry and estimation of nuclear genome size. Annals of Botany 95: 99–110.
Galbraith DW, Harkins KR, Maddox JM (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissue. Science 220: 1049–1051.
Ishigaki G, Gondo T, Suenaga K, Akashi R (2009) Multiple shoot formation, somatic embryogenesis and plant regeneration from seed-derived shoot apical meristems in ruzigrass (Brachiaria ruziziensis). Grassl Sci 55:46–51.
Ishigaki G, Gondo T, Ebina M, Suenaga K, Akashi R (2010) Estimation of genome size of Brachiaria species. Grassl Sci: 56: 240–242.
Larkin PJ, Scowcroft WR (1981) Somaclonal variation-a novel source of variability from cell culture for plant improvement. Theoretical and applied genetics 60: 197–214.
Lutts S, Kinet JM, Bourharmont J (2001) Somaclonal variation in rice after two successive cycles of mature embryo derived callus culture in the presence of NaCl. Biol Planta 44: 489–495.
Phillips RL,Kaeppler SM and Olhoft P (1994) Genetic instability of plant tissue culture. Proc. Natl. Acad Sci. USA 91: 5222–5226.
Uozu S, Ikehashi H, Ohmido N, Ohtsubo H, Ohtsubo E, Fukui K (1997) Repetitive sequences: Cause for variation in genome size and chromosome morphology in the genus Oryza. Plant Molecular Biology 35: 791–799.
37
Discussion and feedback