A NARRATIVE REVIEW OF APIACEAE FAMILY PLANTS IN USADA NETRA FOR EYE DISEASE TREATMENT
on

Journal of Pharmaceutical Science and Application Volume 2, Issue 2, Page 49-65, December 2020 E-ISSN : 2301-7708
A NARRATIVE REVIEW OF APIACEAE FAMILY PLANTS IN USADA
NETRA FOR EYE DISEASE TREATMENT
Luh Pande Putu Tirta1, A.A Gede Rai Yadnya-Putra1*
1Department of Pharmacy, Faculty of Mathematics and Sciences, Udayana University, Bali-Indonesia
Corresponding author email: agungryp@unud.ac.id
ABSTRACT
Background: Eye health really needs to be maintained so as to avoid diseases such as red-eye (conjunctivitis) and stye (hordeolum). Conjunctivitis and hordeolum are caused by bacteria, and their treatment is generally using antibiotics. However, if antibiotics are used irrationally it can be dangerous to health, therefore at this time, people are more interested in traditional medicine. One of them is Balinese traditional medicine, Usada Netra. Usada Netra is a science that deals with the prevention, treatment and recovery of eye diseases. Objective: This review discusses conjunctivitis, hordeolum and its treatment, processing of herbs for eye diseases in Usada Netra, botanical aspects, phytochemical and pharmacological effects of three plant Apiaceae family based on the results of scientific research as well as the correlation with eye treatment in pharmaceutical sciences. Methods: Search data in this article review with the help of search engines namely Google Scholar, and online journal provider sites, including PubMed, Biomed, NCBI, and primary data obtained from international journals, national journals and usada Bali books that have been published. Result: Based on the pharmacological effects produced by the Apiaceae family plant can inhibit the growth of bacteria that cause conjunctivitis and hordeolum. Conclusion: The use of plant Apiaceae family can be recommended for treatment of the eye such as conjunctivitis and hordeolum, with due regard to the safety requirements for eye treatment preparations.
Keywords: Antibacterial, Apiaceae Family, Conjunctivitis, Hordeolum
INTRODUCTION
Eye health is very important to avoid disease. Eye disease is often the case that the red-eye (conjunctivitis) and stye (hordeolum). Conjunctivitis and hordeolum are caused by bacteria, so their treatment is generally using antibiotics[1,2]. However, the irrational/ handling use of an antibiotic can cause bacteria to become resistant, which can certainly be harmful to health. So that now the Indonesian people are increasingly heading towards the paradigm of back to nature by choosing to use natural ingredients to overcome health problems[3].
WHO (World Health Organization) also recommends the use of traditional
medicines or herbal medicines in maintaining public health and for the prevention and treatment of diseases[3]. One of the traditional treatments found in Bali is Usada Bali. Usada Bali is a derivative of Ayurveda. Ayurveda is part of Upaveda, while Upaveda is part of Vedic Smerti coming to Bali in the tenth century in the era of King Udayana Government. The Usada Bali System is basically a Religious Magical Empiricist. The traditional treatment system by WHO is recognized as Traditional Medicine / Complementary and Alternative Medicine (TM / CAM)[4].
One part of Usada Bali is Usada Netra. Usada Netra discusses various diseases that can occur in humans and its treatment. Usada Netra's treatment is based on experience, word or revelation by utilizing plants, mantras and rajahan or paintings to treat various diseases. Usada Netra is a medical treatment for eye diseases[5]. Many plants contained in Usada Netra are useful in the treatment of eye diseases, one of which is from the Apiaceae family.
Apiaceae family is one of the plant families with the most types of plants found and used by the Balinese people. The Balinese use plants from the Apiaceae family such as Fennel (Foenicullum vulgare Mill.), Gotu Kola (Centella asiatica L.), and Coriander (Coriandrum sativum) as a treatment for eye diseases[6,5].
This review discusses of conjunctivitis, hordeolum and its treatment, processing of ingredients for eye diseases in Usada Netra, botanical aspects, phytochemicals and pharmacological effects of the Apiaceae plant based on the results of scientific research and correlations with eye treatment in pharmaceutical science. It is hoped that the review of this article can be scientific information about the plant of the Apiaceae family in Usada Netra, which has the potential as a standardized herbal medicine for eye diseases.
METHODS
The writing of this article review was preceded by searching plants for the treatment of eye diseases in the Usada Netra book, then using the help of search engines to search for plant species and families. Then all plants are grouped into the same family. Traced general review of plants from the source and the pharmacological effects of plants by searching using the help of search engines namely Google Scholar, and online journal provider sites, including PubMed, Biomed, NCBI, and so on. A literature search was carried out with the keywords "antibacterial activity", "antibacterial plant", and "herbal
medicine". Primary data were obtained from international journals, national journals and usada Bali books that had been published. The selection of inclusion criteria for articles/journals except for the reference book of usada Bali is a research article published in the last ten years (20102020).
RESULTS
Review Of Eye Disease And Its Treatment
A. Conjunctivitis
Conjunctivitis is inflammation of conjunctival tissue that can be caused by an invasion of microorganisms, hypersensitivity reactions or degenerative changes in the conjunctiva. The symptoms are caused by vascular dilatation, cellular infiltration and exudation. Patients usually complain of red-eye, conjunctival oedema and excessive secretions[1].
Conjunctivitis can be grouped based on the time of occurrence, namely acute and chronic. Acute conditions that occurrence of symptoms up to four weeks, while the chronic conjunctivitis symptoms are more than four weeks. Conjunctivitis often occurs along or after respiratory infections, and generally, there is a history of contact with patients with viral conjunctivitis[1]. Acute infective conjunctivitis and viral conjunctivitis are more common in adults and men[7].
The pathophysiology that causes conjunctivitis is microorganisms (viruses, bacteria, fungi), allergens, irritants causing the eyelids to become infected so that the eyelids can not be close and open completely so that the eyes become dry and cause conjunctivitis. The most common bacterial causes of acute conjunctivitis are Staphylococcus aureus, Staphylococcus epidermidis, Haemophilus influenzae, Streptococcus pneumoniae, Streptococus viridans, Moraxella catarrhalis and gramnegative bacteria[8,9].
Treatment for Conjunctivitis :
1. Moxifloxacin
Antibiotics are commercially available for the treatment of bacterial conjunctivitis is moxifloxacin. The mechanism of action of Moxifloxacin is by inhibition of topoisomerase II (DNA gyrase) and topoisomerase IV that are needed for replication, bacterial transcription, repair, and bacterial recombination.[10]
Moxifloxacin has been shown to be active against most bacterial isolates, both grampositive, gram-negative or anaerobic bacteria[11].
Ciprofloxacin is a broad-spectrum antibiotic that is used for the treatment of various eye infections. The dose of the drug ciprofloxacin 3 mg / mL for bacterial conjunctivitis is 1-2 drops every 2 hours for two days, then 1-2 drops every 4 hours for the next five days[12,13].
Chloramphenicol 1% ointment applied 3-4 times daily. The mechanism of action as a broad-spectrum antibiotic and strongly inhibits protein synthesis [12,14].
Gentamicin is an antibiotic of the aminoglycoside group has a broad spectrum against gram-positive and gram-negative bacteria. Dose Gentamicin eye drops 0.3%, 1-2 drops into the conjunctival sac every 4 hours[12,15].
Hordeolum is an acute bacterial infection found on the eyelids[2]. Symptoms of hordeolum are painful inflammation of the eyelids and erythematous. Infection in Hordeolum occurs due to thickening, drying, or stasis in Zeis, Moll, or in the Meibomian gland. The Zeis gland secretes sebum and has antiseptic properties that can prevent bacterial growth[17]. The Moll gland produces immunoglobulin A, mucin, and lysosomes which are important in immune defence against bacteria in the eye[18].
There are two types of hordeolum namely external Hordeolum (stye) that occurs due to infection of the superficial sebaceous glands (eyelash follicles) and internal Hordeolum that occurs due to infection of the meibomian gland (acute meibomianitis). External Hordeolum can last one to two weeks, can heal itself. There are reports that about 90% of hordeolum is caused by Staphylococcus aureus infection[19].
Treatment for Hordeolum :
1. Warm compresses
External hordeolum can be treated with warm compresses and massage therapy. This warm compress is intended to soften the granulomatous tissue and facilitate drainage by removing eyelashes). Special attention is needed when compressing and massaging internal hordeolum, as this can cause irritation or deformation of the cornea[20].
Topical antibiotic use can also be indicated for the treatment of Hordeolum. Persistent or larger lesions may require antibiotic therapy. This treatment can help shorten the duration and severity. Macrolide antibiotic ointments such as erythromycin ophthalmic ointment can be used for 7-10 days[21,2].
If the swelling enlarges and causes pressure on the cornea, it can be treated with topical steroids that are used for a short duration of 2-3 days. If the infection spreads and develops into a periorbital or orbital cellulitis, systemic antibiotics is required[22, 17] .
Usada Netra
Usada Netra is a medical science for eye diseases. In usada Netra there are approximately 17 types of plants for the treatment of eye diseases, swollen eye disease, red eyes, itchy eyelids, eye discharge, and blurred eyes[5]. Many plant families are contained in Usada Netra such as Fabaceae, Euphorbiaceae, Rubiaceae, Amaryllidaceae, Acoraceae, Lamiaeae, Rutaceae, Acanthaceae, Anacardiace, Apocynaceae and also another family plant with the most plants in the Apiaceae family. The following table discusses the Apiaceae tribal plants for eye treatment in Usada Netra, which can be seen in Table 1.
Plant Review
-
1. Fennel (Foenicullum vulgare)
-
a. Botanical Aspects of Fennel
(Foenicullum vulgare)
Fennel (Foeniculum vulgare) is an annual herbaceous plant originating from the Mediterranean and Southern Europe. Fennel grows wild on the coastlines of the Mediterranean, and Egypt. This plant is cultivated throughout the world. Fennel is
divided into two subspecies, namely piperitum and capillaceum and includes three varieties, namely var. vulgare (Mill.), Thell., var. Dulce (Mill.) Thell, and var. Azoricum (Mill.) Thel[23]. Following is the taxonomy of Fenniculum vulgare which can be seen in Table 2[24].
The fennel plant is known by different names in each region as in India under the name Fennel, Canada: Doddasompu, China: Hui xiang, England: Bitter Fennel, Japan: Fenneru, Korea: Sohoehyang, and Indonesia: Adas[24] Fennel (Foeniculum vulgare Mill) has small yellow flowers on stalks are small, thin green leaves downy can grow up to 40 cm and a width of about 0.5 mm, hollow stems, dried fruit with a length of 4-10 mm. Fennel can grow to a height of 2.5 m[25.26]. Fennel plants are shown in Figure 1.
Fennel has been widely used in traditional medicine for various diseases. Fennel is used in various traditional systems such as Ayurveda, Unani, Siddha, in India, and Iran's traditional system as an alternative and complementary medicine[27]. Fennel plant in the area of Turkey and Pakistan used as a drug for myopic eyes and itching[28,29].
Fennel contains saponins, flavonoids, cardiac glycosides, sterols, triterpenes, coumarin and essential oils. Fennel contains 42.3% carbohydrates, 18.5% fiber, 13.4% minerals, 10% fat, and 9.5% protein.
Table 1. Overview of Apiaceae for the Treatment of Eyes in the Usada Netra
|
Name of Plant |
Use How to make and use |
|
Fennel (Foeniculum vulgare) |
Treatment of common As much as 3 seeds of Fennel are added with 3 pieces of eye pain apite wood leaves then crushed. How to use it smeared on (conjunctivitis) the eyelid[6]. |
|
Gotu Kola (Centella asiatica L.) |
Treatment of red eyes paiduh leaves (gotu kola) were crushed and dropped into and swollen eyes the eyes 3-4 times a day[5]. (conjunctivitis) |
|
Coriander (Coriandrum sativum L. ) |
Treatment of Coriander, white kecamcem, pulasari, sindrong wayah, “ketumbuhan” miana cemeng, and palm sugar, are processed into loloh (hordeolum) (to be drunk), then the pulp is used to boreh (smeared) on the skin of the diseased eyelid[6]. |
Table 2. Taxonomy Fennel (Foeniculum vulgare)
Nomenclature of Fennel (Foeniculum vulgare)
|
Kingdom |
Plantae |
|
Division |
Tracheophyta |
|
Sub-division |
Spermatophytina |
|
Class |
Magnoliopsida |
|
Order |
Apiales |
|
Family |
Apiaceae |
|
Genus |
Foeniculum |
|
Species |
Foeniculum vulgare Mill |
The minerals and vitamins contained in Fennel are phosphorus, niacin, iron, potassium, calcium, sodium, thiamine, riboflavin, and vitamin C[30,31].
Flavonoids are phenolic compounds that are strong antioxidants. The total phenolic content in the Fennel essential oil is 262.59 ± 15.5 mg. Fennel is also famous for its essential oil content. The main components of Fennel essential oils are trans-anethole, fenchone, estragole (methyl chavicol), and alpha-phellandrene[32,33,25]. Following is the chemical structure of Fennel essential oils in Figure 2.

(a) (b)

(c) (d)
Figure 1. Fennel plants (a) in native habitat. (b) Flowers, (c) Fruits, (d) Seeds[24]
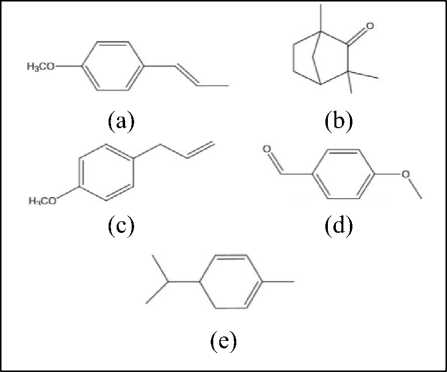
Figure 2. Molecular structure of essential components of Fennel oil. (a) Trans-anethole, (b) Fenchone, (c) Estragol, (d) Para-anisaldehyde, (e) Alpha-phellandrene[25]
The antibacterial effects of methanolic extracts of 23 fennel samples were evaluated against many bacterial isolates. The seed extracts of two samples showed moderate to good inhibitory activities (MIC: 62.5-125μg/ml) against three bacteria[35]. The fennel seed extract was tested for antimicrobial activity against Staphylococcus aureus, Micrococcus spp and Entecococcus spp. The results showed that the ethanol extract of Foeniculum vulgare had greater activity against Micrococcus spp. (MIC = 250μg / ml)[36].
Antimicrobial effects of methanol, ethanol, diethyl ether, and hexane extracts from Foeniculum vulgare seeds were also tested against Escherichia coli, Salmonella typhi, Bacillus cereus, Staphylococcus aureus, Candida albicans and Aspergillus flavus. Extracts with methanol solvents showed higher antimicrobial activity than other extracts. The results showed that Bacillus cereus and Aspergillus flavus were the most sensitive microorganisms, showing the largest inhibitory zone and the lowest MIC value. The least recorded antimicrobial activity against Escherichia coli[33].
The antibacterial activity of the essential oil of Fennel was tested against three Gram-positive bacteria namely Staphylococcus epidermidis, Enterococcus faecalis, and Staphylococcus aureus and six Gram-negative bacteria namely Morganella morganii, Escherichia coli, Proteus mirabilis, S.enteritidis serovar Typhimurium, Salmonella enteritidis, and Psemorosa infusion. The results showed the essential oil of Fennel proved effective against antibiotic-resistant strains, namely Enterococcus faecalis, Escherichia coli, M. morganii, Salmonella enteriditis and S. centiritidis serovar Typhimurium. Fennel essential oils also have antibacterial activity against S.epidermidis, S. aureus, although the level of inhibition is still low when compared with antibiotics ampicillin (10 μg), cephalothin (30 μg), and tetracycline (30 μg)[37].
Besides having been tested for Fennel's pharmacological activity as an antibacterial, Fennel also has anti-fungal activity. The in vitro antifungal activity of the essential oil Foeniculum vulgare was investigated for three strains of Candida albicans from different places using disks, the well diffusion method, microdilution, and compared with standard antimycotics drugs namely Nystatine and Fluconazole. The results showed that the essential oils studied showed antifungal activity against all C. albicans isolates (MIC value: 0.06 mg / ml - 0.23 mg / ml)[38].
Antioxidant molecules capable of correcting the oxidative stress imbalance are becoming increasingly crucial to the prevention and treatment of various eye diseases. Eye drops made from Foeniculum vulgare seed water extract have a protective and therapeutic effect in rats induced by sodium selenite, which causes cataracts. This is seen in decreasing the lens's turbidity score and increasing scanning electron microscopy. The antioxidant effect of Foeniculum vulgare extract allows for
therapeutic and protective effects on the eyes[39].
Fennel extract (70% ethanol) at doses of 100 and 200 mg/kg given for 5 days, intraperitoneally in female mice, the results showed that Fennel extract could reduce serum levels of oxidative factors in female rats. [40] The antioxidant activity of essential oils contained in Fennel is measured in terms of the ability to contribute to hydrogen or radical cleaning, using DPPH. Fennel essential oil showed radical cleansing ability at all concentrations tested, % Inhibition DPPH 11.24-21.88, IC50: 45.89g / l. Fennel ferric oil reduction capacity of 0.19-0.37 mmol / l Trolox[32].
A methanol extract of Foeniculum vulgare showed the highest analgesic / antinociceptive activity at a dose level of 2000 mg/kg, whereas the activity indicated by ethyl acetate extract was (800 mg/kg). On the other hand, n-hexane extract (700 mg/kg) and methylene chloride extract (500 mg/kg) showed the same antinociceptive activity, when compared with acetylsalicylic acid (200 mg/kg) activity was lower. The results also revealed that the extract investigated showed significant anti-inflammatory activity. The methanol extract of Foeniculum vulgare has the highest activity, which significantly reduces the weight of oedema caused by carrageenan in rat feet at dose levels of 1500 and 2000 mg/kg, each having a protective effect of 28 and 47%, compared to the control value, while ibuprofen (35 mg/kg), used as a standard drug shows a protective effect of 52.23%. Anti-inflammatory effects of Fennel can restore the body's homeostasis so as to prevent and treat eye diseases[41].
-
2. Gotu Kola (Centella asiatica L.)
-
a. Botanical Aspects of Gotu Kola (Centella asiatica L.)
Centella asiatica is found throughout the tropics and sub-tropics of India. Centella asiatica can grow up to 600m tall. This plant originates from Southeast Asia, India and Sri Lanka, parts of China, Madagascar, South Africa, the Southeast United States, Mexico, Venezuela, Colombia, and Eastern South America. Centella asiatica L. is a medicinal plant that has long been used by the people of Java and Indonesia. Centella asiatica (L.) is known by various different names in each region such as Thankuni (Bengali), Mandukaparni (Hindi), Pegga (Malay), Gotu kola (Sinhala), Gedarai (Tamil), and Bekasianam (Telugu)[42,43]. The following is a taxonomy of Centella asiatica L. which can be seen in Table 3.
Table 3. Taxonomy of Gotu kola (Centella asiatica L.)[45]
Nomenclature of Gotu kola plant (Centella asiatica L.)
|
Kingdom |
Embryophyta |
|
Sub-kingdom |
Spermatophyta |
|
Division |
Spermatophyta |
|
Sub-division |
Angiospermae |
|
Class |
Dicotyledonae |
|
Sub-class |
Rosidae |
|
Sub-order |
Aralianae |
|
Order |
Araliales |
|
Family |
Apiaceae |
|
Sub-family |
Hydrocotyle |
|
Genus |
Centella |
|
Species |
Centella Asiatica L. |
Centella asiatica (L.) is a plant that grows vines, slightly scented, spreads, and can grow to heights of up to 15 cm (6 inches). Centella asiatica thrives in shady, swampy, damp and wet areas such as rice fields, and can also grow on sandy loam soils. Centella asiatica (L.) is found in fertile soils for regeneration. The trunk is bald, striated. The leaves, 1-3 from each stem knot, stem length 2-6 cm and width 1.5-5 cm, base of leaf sheath, hairy on both sides. The flowers are beautifully shaped umbles, each umbel consisting of 3-4 white to purple or pink flowers. Centella asiatica L. usually blooms in April-June. The fruit is about 2 inches long, oval, round and very
thick pericarp[42,44]. The following is a Gotu kola plant shown shown in Figure 3.

(a)
(b)
Figure 3. Gotu kola plants, (a) with flowers, (b) with roots[45]
The main active compounds of Centella asiatica (L.) are pentacyclic triterpenes such as asiatic acid, madecassic acid, asiaticoside and madecassoside[46,47]. Quantification of Centella asiatica (L.) triterpenes has been successfully determined by some researchers using HPLC-UV. However, the triterpene component in Centella asiatica is known to vary depending on the conditions and location of its growth[48]. Following is the chemical structure of the triterpene content of Centella asiatica (L.) which can be seen in Figure 4.
Gotu kola plants consist of phenylpropanoids phenols such as rosmarinic acid, chlorogenic acid, 3,4-di-o-caffeoyl quinic acid, 1,5-di-o-caffeoyl quinic acid, 3,5-di-o-caffeoyl quinic acid, 4,5-di-o-caffeoyl quinic acid, isochlorogenic acid, tannin, and phylactic. [49] In addition, Centella asiatica (L.) also contains flavonoids with high total phenolic content such as quercetin, kaempherol, catechin, rutin, apigenin and naringin and essential oils such as caryophyllene, farnesol and elements. P-cymene- (44%) and many other volatile compounds in Centella asiatica (L.) essential oils in analysis by GC-MS[50].
Centella asiatica (L.) is also rich in vitamin C, vitamin B1, vitamin B2, niacin, carotene and vitamin A. The total ash content of Centella asiatica (L.) contains chloride, sulfate, phosphate, iron, calcium, magnesium, sodium and potassium[51].
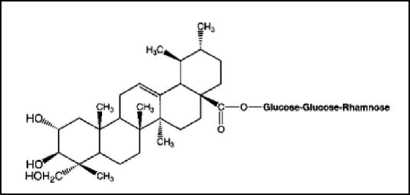
(a)
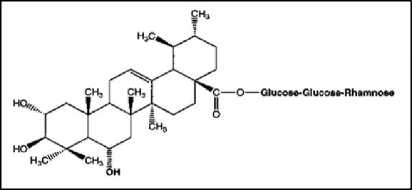
(b)
Figure 4. The chemical structure of Centella asiatica (L.). (a) Asiaticoside and asiatic acid (without GlucoseRhamnose); (b) Madecassoside and madecassic acid (without Glucose-Rhamnose[48].
Gotu kola plants were cultured on leaf explants with MS media and tested for their antibacterial activity against B. cereus, E. coli, S. aureus, and P. aeruginosa, which produced methanol extracts of leaves and callus which had maximum inhibitory effects on the organisms tested[52]. Antibacterial activity tests can also be carried out with the method so that the largest diffusion well and disc plate inhibition zone (10.0 mm) is shown by Centella asiatica L. root ethanol extract against S. aureus while the values drop to 5.0 and 3.5. mm for extracts with chloroform solvents and water extracts when tested against S. aureus[53].
Eye disease can also be caused by invasion from fungi[8,9]. Antifungal activity test Centella asiatica L. has been carried out on several types of fungi. The ethanol extract of Centella asiatica L. was tested for antifungal activity against Aspergillus flavus, and Penicillium citrinum showed the
strongest antimold activity (percentage of mycelia inhibition = 26.3 mm)[54]. The ethanol extract activity of 100% Centella asiatica L. shows a zone of inhibition of 15.4 mm against A. niger. [55] The antifungal activity of Centella asiatica L. was also tested against Candida albicans, with an average inhibition zone yield of 5 mm, while the standard zone of miconazole nitrate inhibition showed 20 mm inhibition[56].
Anti-inflammatory effects can inhibit pathological conditions and restore the body's homeostasis so as to prevent and treat eye diseases. Centella asiatica L. can inhibit the damage to human red blood cell membranes. At different concentrations, membrane stabilization was observed for diclofenac sodium and methanol extracts, and at a dose of 2000 μg / ml, the maximum membrane stabilization of Centella asiatica L extract was recorded at 94.97%[57]. The asiatic acid content of Gotu kola was tested at the 4th and 5th hours, after the addition of λ-carrageenan (Carr), it was found that the asiatic acid content could reduce foot edema in mice through increased regulation of catalase activity, superoxide dismutase (SOD), and glutathione (GPx) in liver tissue[58].
The asiaticoside content tested in lipopolysaccharide-induced mice obtained results in inhibition of the fever process and inflammatory response, including serum TNF-α and IL-6 production, liver myeloperoxidase (MPO) activity, COX-2 brain protein expression and prostaglandin production[59]. The asiaticoside G content is also reported to have anti-inflammatory properties on RAW 264.7 cells stimulated by lipopolysaccharides (LPS)[60].
Gotu kola plant extracts obtained from Turkey and India compared to standard plant extracts obtained from China. Three extracts at concentrations of 250, 500, 1000,
and 2000 μg / mL showed radical scavenging activity for testing at 2,2-diphenyl-1-picrillhidrazil (DPPH)[61].
Polyphenolic compounds, flavonoids, β-carotene, tannins, vitamin C, which are found in Centella asiatica L significantly have antioxidant activity. The antioxidant activity of gotu kola can also improve oxidative stress which is important for the prevention and treatment of eye diseases[62].
Coriandrum sativum L. is a herbal plant that has nutritional value and is used as a spice that is useful as traditional medicine[63,64]. Coriandrum sativum L. is believed to have originated in the Mediterranean and is known by various names such as in Arabic: kuzbara, Chinese: yuan sui, hu sui, Dutch: Coriander, English: coriander, collender, Chinese: parsley, Indian: dhania, Japan: Koendoro and
Malaysia: Coriander[65]. The following is the taxonomy of the Coriander (Coriandrum sativum L.) plant in Table 4.
Table 4. Taxonomy of coriander (Coriandrum sativum L.)[65]
The nomenclature of Coriander (Coriandrum sativum L.)
|
Kingdom |
Plantae |
|
Subkingdom |
Viridaeplantae |
|
Division |
Tracheophyta |
|
Class |
Magnoliopsida |
|
Order |
Apiales |
|
Family |
Apiaceae |
|
Genus |
Coriandrum |
|
Species |
Coriandrum sativum L.) |
Coriandrum sativum L. is a herbal plant that has nutritional value and is used as a spice that is useful as traditional medicine[63,64]. Coriandrum sativum L. is believed to have originated in the Mediterranean and is known by various names such as in Arabic: kuzbara, Chinese: yuan sui, hu sui, Dutch: Coriander, English: coriander, collender, Chinese: parsley, Indian: dhania, Japan: Koendoro and Malaysia: Coriander[65].
Coriandrum sativum L. is an annual plant, has taproots, and slender branched stems can grow to 20-70 cm tall. There are two varieties of Coriandrum sativum L.: vulgare and microcarpum; Vulgare has larger fruit (3-5 mm diameter) by producing essential oil 0.1% -0.35% (v / w) while microcarpum has smaller fruit (1.5-3 mm diameter) by producing oil volatile 0.8% -1.8% (v / w). The leaves are lanceolate, green or dark green, and have varying shapes and lobes. The flowers grow in small umbles, white or pink, asymmetric. Dried schizocarp coriander fruit is almost ovoid with two mericarps and has a sweet, slightly pungent taste[66,67]. The following is a picture of coriander plants that can be seen in Figure 5.

(a) (b) (c)
Figure 5. Coriander plants. (a) Coriander Leaves, (b) Coriander Fruit, (c) Coriander Flowers[65,68].
Coriandrum sativum L. contains bioactive components such as flavonoids (quercetin and isoquercetin, rutin), terpenes (linalool), terpenoids, polyphenols (ferrulic acid, gallic acid, rutin, caffeic acid, anethole, borneol, carotenoids), fatty acids rich in petroselinic acid, coumarin and hydroxy-coumarin, tannins, vitamin C sterols and tocopherols[63,69]. Following is a table of the composition of the chemical compound of Coriandrum sativum L. essential oils seen in Table 5.
Based on the table above it can be seen that the highest chemical content in the alcohol group is Linaloolm hydrocarbons, namely g-terpinene, ketones namely Camphor and Esters, namely Geranyl
acetate. 90% of essential oils which were identified using GC-FID and GC-MS showed that Linalool was the most commonly found component at 69.60%.
Table 5. Chemical compound and composition of Coriandrum sativum L. essential oil[69]
Chemical Composition
compound
Alcohols Linalool (60–80%), geraniol
(1.2%–4.6%), terpinen-4-ol (3%), a-terpineol (0.5%)
Hydrocarbons g-terpinene (1–8%), r-cymene (3.5%), limonene (0.5%– 4.0%), a-pinene (0.2%–8.5%), camphene (1.4%), myrcene (0.2%–2.0%)
Ketones Camphor (0.9%–4.9%)
Ester Geranyl acetate (0.1%–4.7%),
linalyl acetate (0%–2.7%)
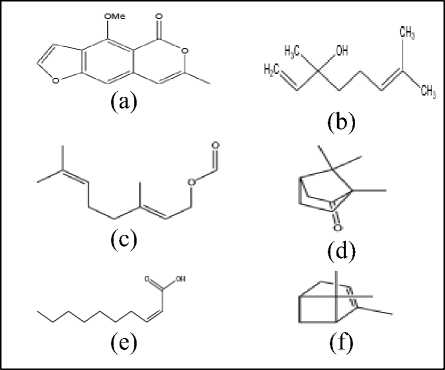
Figure 6. Chemical Structure of Coriandrum sativum L. (a) Coriandrin, (b) Linalool, (c) Geranyl acetate, (d) Camphor, (e) 2-decenoic acid, (f) Alpha pinene[68,71].
Other ingredients found were geranyl acetate (4.99%), ter-terpinene (4.17%), alpha-pinene (1.63%), anethol (1.15%) and p-cymene (1.12% )[70]. The following is the chemical structure of the compounds contained in Coriandrum sativum L. shown in Figure 6.
The essential oil of C. sativum L. Effective antibacterial activity against Staphylococcus aureus and Gram-negative bacterial strains including E. coli, Klebsiella pneumoniae, Salmonella typhimurium, and P. aeruginosa as well as two Acinetobacter baumannii strains that are clinically resistant multi drug. The main mechanism of action of Coriander essential oil is to damage the cell membrane of bacteria, causing cell death[72]. Test antibacterial activity with different extraction solvents showed that only aqueous extract of C. sativum L has stable antibacterial activity under heating and has the best antibacterial effect at pH 6 with a concentration of NaCl 2.0%[73].
Test the activity of C. sativum L. essential oils in different bacterial isolates using the microdilution method. Essential oils of Coriandrum sativum L. showed good antibacterial activity against most strains of bacteria tested, including Streptococcus pyogenes (Lancefield group A) and methicillin-resistant Staphylococcus aureus (MRSA), with a minimum average inhibitory concentration of 0.04 respectively % v / v and 0.25% v / v[74]. Coriandrum sativum L. essential oil activity was tested using a different method, namely the Kirby-Bauer disk diffusion method. Coriandrum sativum L. had a lower antibacterial activity for gram-positive bacteria than gram-negative bacteria[75].
Antibacterial activity against grampositive namely Staphylococcus aureus obtained inhibition zone diameter of (8.67 ± 0.32) mm when compared with Ampicillin (10.37 ± 0.30) mm and Gentamicin (10.37 ± 0.25), while the highest activity against E. coli (10.73 ± 0 , 21) mm compared with the Gentamicin antibiotic (9.47 ± 0.45) mm. Coriander also inhibits the growth of Salmonella typhii (9.53 ± 0.40) mm which is slightly lower than Ampicillin (10.57 ± 0.21) mm and has little antibacterial activity against Klebsiella (7.20 ± 0.17) mm but
close to the antibacterial activity of ampicillin antibiotics (8.43 ± 0.25) mm[75]. The following table compares the antibacterial activity test of Coriandrum sativum L. essential oils which can be seen in Table 6.
Based on the pharmacological effects of the Apiaceae plants produced, namely Fennel (Foenicullum vulgare Mill.), Gotu kola (Centella asiatica L.) and Coriander (Coriandrum sativum) all plants have antibacterial activity, which bacteria are a common cause of conjunctivitis and hordeolum eye disease.
Table 6. Comparison of inhibition zones Coriander, ampicillin, and gentamicin essential oils in different microorganisms[75]
Inhibition zone (mm)
|
Microor ganisms |
Essential oil of Coriander |
Ampicillin |
Gentamicin |
|
S.aureus |
8.67±0.32 |
10.37±0.30 |
10.37±0.25 |
|
E. Coli |
10.73±0.21 |
11.37±0.21 |
9.47±0.45 |
|
K. |
7,20 ± 0,17 |
8,43 ± 0,25 |
10.83±0.12 |
|
pneumon iae Pseudom |
8.33±0.29 |
11.53±0.21 |
9.67±0.40 |
|
onas S. typhi |
9,53 ± 0,40 |
10,57±0,21 |
12.50±0.20 |
DISCUSSION
The eye is an organ that is vital for life. Eye health is very important to avoid disease. An eye disease that often occurs is red-eye (conjunctivitis) and stye (Hordeolum). Conjunctivitis is an inflammation of conjunctival tissue that can be caused by the invasion of microorganisms. The most common bacteria that cause acute conjunctivitis are S. aureus, Staphylococcus epidermidis, H. influenzae, Streptococcus pneumoniae, Streptococus viridans, Moraxella catarrhalis and gram-negative bacteria. Hordeolum is an acute bacterial infection found in the eyelid. The bacterium that causes hordeolum is Staphylococcus aureus[9,2,19].
The use of synthetic drugs, such as antibiotics can reduce the effects caused by conjunctivitis or hordeolum. Antibiotics that can be used for the treatment of conjunctivitis are Ciprofloxacin, and for the treatment of Hordeolum, one of them can use erythromycin. [12,2] But the use of antibiotics that are irrational/improper can cause bacteria to become resistant, which can certainly be harmful to health. So many people have switched to using plants as herbal medicines. Herbal medicine is used for treatment throughout the world because it has been tested to have activity as an antimicrobial, immunomodulatory, which can prevent or treat diseases, both animal or human[76].
One of the rich culture of the community regarding traditional medicine using herbs is found in Usada Netra. Usada Netra is derived from the word "usada" meaning healing and "netra" means eyes or vision, Usada Netra is a medical treatment for eye diseases[6].
Many plants contained in Usada Netra play a role in the treatment of eye diseases; one of them is the Apiaceae plant. The Apiaceae is the most family species found and used by the Balinese people. Balinese people make use of the plants of the Apiaceae family such as Fennel (Foenicullum vulgare Mill.), Gotu kola (Centella asiatica L.) and Coriander (Coriandrum sativum) for treatment of eye diseases such as red eyes and hordeolum[6,5].
Normal eye disease, red eye or swelling in pharmaceutical terms can be associated with conjunctivitis, which can be caused by an invasion of microorganisms such as viruses, bacteria, or fungi. The bacteria that most commonly cause acute conjunctivitis are S. aureus, Staphylococcus epidermidis, H. influenzae, Streptococcus pneumoniae, Streptococus viridans, Moraxella catarrhalis and gram-negative bacteria[9].
Fennel plants in Usada Netra are used for the treatment of ordinary eyes. [6] Fennel is used to treating many bacterial, fungal,
viral, and mycobacterial infections. [35] The content of essential oil of Fennel has antibacterial activity against S.epidermidis, S. aureus, although the level of inhibition is still low when compared with antibiotics ampicillin, cephalothin, tetracycline. Fennel also has anti-fungal activity. Fennel essential oil also has antifungal activity against all C. albicans isolates (MIC value: 0.06 mg / ml - 0.23 mg / ml)[37,38].
The antioxidant activity test of Foeniculum vulgare extract with Fennel extract has therapeutic and protective effects on the eyes. This is seen in the decrease in lens turbidity score and increase scanning electron microscopy. In addition, Fennel also has anti-inflammatory and analgesic activity. The fennel antioxidant activity can reduce the destructive cellular consequences of oxidative stress that are important for the prevention and treatment of eye diseases[31,39,41]. So it can be concluded that the Fennel plant can be utilized for the treatment of eye diseases, namely Conjunctivitis (common eye disease) as stated on Usada Netra.
Gotu Kola (Centella asiatica L.) in Usada Netra is used for the treatment of red and swollen eyes[5]. The ethanol extract of Centella asiatica L. root has antibacterial activity with the biggest inhibition zone yield (10.0 mm) against S. aureus bacteria. In addition, Centella asiatica L. ethanol extract was tested for antifungal activity against Aspergillus flavus, and Penicillium citrinum showed the strongest antifungal / antimold activity (percentage of mycelia inhibition = 26.3 mm)[53,54]. Gotu kola (Centella asiatica (L.) also has antiinflammatory and antioxidant activity. The antioxidant activity of gotu kola can also improve oxidative stress which is important for the prevention and treatment of eye diseases. [60,61] So based on the pharmacological effects produced above it can be concluded that Centella asiatica (L.) can be used for the treatment of eye diseases namely conjunctivitis (red and swollen
eyes) according to those listed on Usada Netra.
Coriander plants (Coriandrum sativum L.) in Usada Netra are used to treat the eyes that come out of plants (stye or hordeolum). Hordeolum is an acute bacterial infection found in the eyelid where the bacterium that causes hordeolum is Staphylococcus aureus[19,2]. The essential oil of Coriandrum sativum L. shows good antibacterial activity against most strains of bacteria tested, including Streptococcus pyogenes (Lancefield group A) and methicillin-resistant Staphylococcus aureus (MRSA), with a minimum average inhibitory concentration of 0.04 respectively % v / v and 0.25% v / v. [74] So based on the pharmacological effects produced above it can be concluded that Coriandrum sativum L.) can be used for the treatment of eye diseases, namely Hordeolum (plant on the eye) in accordance with those listed on Usada Netra.
The use of traditional plants as eye medicine must not be carelessly fulfilled the requirements of sterile preparations for the eyes. Making sterile preparations for the eyes must be done aseptically. The aseptic method is a working technique that attempts to prevent and avoid contamination from microorganisms and reduce the risk of exposure from dosage makers[77]. Sterile preparations for eyes have the requirements to be sterile, free from microbial contaminants or foreign matter, and must be free of particles that can irritate the eye[78].
CONCLUSION
Based on the review above it can be concluded that the plants of the Apiaceae family namely Fennel, Gotu Kola and Coriander which are listed in Usada Netra have pharmacological activities that have been tested as antibacterial, antioxidant, analgesic antifungal and anti-inflammation. So the use of plant Apiaceae family can be recommended for treatment of the eye such as conjunctivitis and hordeolum, but with due regard to the safety requirements for
eye treatment preparations. And it is hoped that the potential of the Apiaceae family plant contained in Usada Netra can be used as a standardized herbal medicine for eye diseases.
CONFLICT OF INTEREST
This paper was written independently. All authors disclose no financial or personal relationships with other people or organizations that could inappropriately influence the work.
ACKNOWLEDGMENT
We thank to lecturers and staff in the Department of Pharmacy, Udayana University, Bali, for the support in the implementation of research.
REFERENCE
-
1. Azari AA and Barney NP. Conjunctivitis: A systematic Review of Diagnosis and Treatment. Journal American Medical Association. 2013; 310 (16): 1721–1729.
-
2. Lindsley K, Nichols JJ, and Dickersin K. Non-surgical interventions for acute internal hordeolum. Cochrane Database Syst Rev. 2017; (1): CD007742.
-
3. Katno. Tingkat Manfaat, Keamanan, dan Efektifitas Tanaman Obat dan Obat Tradisional. Tawangmangu: Badan
Penelitian dan Pengembangan
Kesehatan Departemen Kesehatan RI; 2008.
-
4. Suatama IB. 2019. Multikulturalisme Usada Bali. Jurnal Widya Kesehatan. Universitas Hindu Indonesia. 2019; 1 (1): 1-9.
-
5. Dinkes Prov. Bali. 2008. Himpunan Usada I. Denpasar: UPTD B POT KOM; 2008
-
6. Nala N. Usada Bali. Denpasar: PT. Upada Sastra; 1992.
-
7. Shrestha1 SP, Khadka, Pokhrel AK, and Sathian B. Acute bacterial conjunctivitis – antibiotic susceptibility and resistance to commercially available topical antibiotics in Nepal.
Nepal J Ophthalmol. 2015; 8 (15): 2335.
-
8. Sitompul R. Konjungtivitis Viral : Diagnosis dan Terapi di Pelayanan Kesehatan Primer. Journal Kedokteran Indonesia. 2017; 5 (1): 64–71.
-
9. Abdurrauf M. Memutus Mata Rantai Penularan Konjungtivitis Bakteri Akut. Idea Nursing Journal. 2016; 7(2): 62-65.
-
10. Petri WA. Chapter 52: sulfonamides, trimethoprim-sulfamethoxazole, quinolones, and agents for urinary tract infections, in: B.A. Chabner, B.C. Knollmann, L.L. Brunton (Eds.), The Pharmacological Basis of
Therapeutics.12th ed. New York: Goodman & Gilman’s; 2011.
-
11. Omari MMH, Jaafari DS, Al-Shou’od KA, and Badwan AA. Moxifloxacin Hydrochloride. Burlington: Academic Press; 2014.
-
12. Junaid I. Pedoman Praktis Obat Indonesia. Jakarta: PT. Bhuana Ilmu Populer; 2012.
-
13. Khan A, Iqbal Z, Khan MI, Khan JA, Javed MK, and Ahmad Z. Drug-Drug Interaction Between Ciprofloxacin and Diclofenac Ophthalmic Drops at Ocular Level. African Journal of Pharmacy and Pharmacology. 2011; 5(23): 2566-2574
-
14. Shukla P, Bansode FW, and Singh RK. Chloramphenicol Toxicity: A Review. Journal of Medicine and Medical Sciences 2011; 2(13): 1313-1316.
-
15. Radigan EA, Gilchrist NA, and Miller MA. Management of aminoglycosides in the intensive care unit. JIC. 2010; 25(6):327– 342.
-
16. Turan A, Saricaoglu H, Baskan EB, Turan H, and Aydogan K. Efficacy of Topical Sodium Sulfacetamide in the Treatment of Mild and Moderate Acne Vulgaris: A Randomized, Comparative Study. Turkderm. 2012; 46(8): 33-26.
-
17. Pflipsen M, Massaquoi M, and Wolf S. Evaluation of the Painful Eye. Am Fam Physician. 2016; 93(12): 991-998.
-
18. Takahashi Y, Watanabe A, Matsuda H, Nakamura Y, Nakano T, Asamoto K,
Ikeda H, and Kakizaki H. Anatomy of secretory glands in the eyelid and conjunctiva: a photographic
review. Ophthalmic Plast Reconstr Surg. 2013; 29(3):215-219.
-
19. Bharathi MJ, Ramakrishnan R, Shivakumar C, Meenakshi R, and Lionalraj D. Etiology and antibacterial susceptibility pattern of community-acquired bacterial ocular infections in a tertiary eye care hospital in South India. Indian Journal of Ophthalmology. 2010; 58(6):497–507.
-
20. McMonnies CW, Korb DR, Blackie CA. The role of heat in rubbing and massage-related corneal
deformation. Cont Lens Anterior Eye. 2012; 35(4):148-154.
-
21. McAlinden C, González-Andrades M, and Skiadaresi E. Hordeolum Acute abscess within an eyelid sebaceous gland. Cleve Clin J Med. 2016; 83(5):332-334
-
22. Jin KW, Shin YJ, and Hyon JY. Effects of chalazia on corneal astigmatism : Large-sized chalazia in middle upper eyelids compress the cornea and induce the corneal astigmatism. BMC
Ophthalmol. 2017; 17(36); 1-9.
-
23. Napoli EM, Curcuruto G, and Ruberto G. Screening the Essential Oil Composition of Wild Sicilian Fennel. Biochemical Systematics and Ecology. Elsevier Ltd. 2010; 38(2): 213–23.
-
24. Badgujar SB, Vainav, Patel, and Bandivdekar AH. Foeniculum vulgare Mill: A Review of Its Botany,
Phytochemistry, Pharmacology,
Contemporary Application, and Toxicology. BioMed Research International. 2014; 1-32.
-
25. Rather MA, Dar BA, Sofi SN, Bhat BA, and Qurishi MA. Foeniculum Vulgare: A Comprehensive Review of Its Traditional Use, Phytochemistry, Pharmacology, and Safety. Arabian Journal of Chemistry. 2012; 9(2): 157483.
-
26. Singh SP. A Comprehensive Review on Pharmacological Activity of
Foeniculum vulgare. Global Journal of Pharmacy & Pharmaceutical Sciences. 2019; 7(1); 1-5.
-
27. Rahimi R, and Ardekani MRS.
Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chinese Journal of Integrative Medicine. 2013; 19(1): 73–79.
-
28. Mahmood A, Mahmood A, Malik RN, and Shinwari ZK. Indigenous
knowledge of medicinal plants from Gujranwala district, Pakistan. Journal of Ethnopharmacology. 2013; 148(2):
714–723.
-
29. Polat R, and Satil F. An ethnobotanical survey of medicinal plants in Edremit Gulf (Balikesir, Turkey). Journal of Ethnopharmacology. 2012; 139 (2):
626–641.
-
30. AL-Snafi AE. The chemical constituents and pharmacological effects of Foeniculum vulgare- A review. IOSR Journal Of Pharmacy. 2013; 8(5): 81-96.
-
31. Elizabeth AA, Josephine G, Muthiah NS and Muniappan M. Evaluation of analgesic and anti-inflammatory effect of Foeniculum vulgare. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2014; 5(2):
658-668.
-
32. Marin I, Sayas BE, Viuda MM, Navarro C and Sendra E. Chemical composition, antioxidant and antimicrobial activity of essential oils from organic Fennel, parsley, and lavender from Spain. Foods 2016; 5: 1-8.
-
33. Roby MH, Sarhan MA, Selim KA, and Khalel. Antioxidant and antimicrobial activities of essential oil and extracts of Fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Industrial Crops and Products. 2013; 44: 437-455.
-
34. Amar Z, Noureddine G, Ahmed E, Mohamed FH, Tarik AM, Ahmed T, et
al. Chemical Constituents From Algerian Foeniculum vulgare Aerial Parts And Evaluation Of Antimicrobial Activity. J.Chil.Chem.Soc. 2011; 56(3) : 759-763.
-
35. Salami M, Rahimmalek M and Ehtemam MH. Inhibitory effect of different fennel (Foeniculum vulgare) samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem 2016;213:196-205.
-
36. Rocha DK, Matosc O, Novoa MT, Figueiredo AC, Delgado M and Moiteiro C. Larvicidal activity against Aedes aegypti of Foeniculum vulgare essential oils from Portugal and Cape Verde. Nat Prod Commun. 2015; 10(4): 677-682.
-
37. Mota AS, Martinsb MR, Arantesb S, Lopesc VR, Bettencourtd E, Pombala S, Gomesa AC and Silvaa LA.
Antimicrobial Activity and Chemical Composition of the Essential Oils of Portuguese Foeniculum vulgare Fruits. Natural Product Communications. 2015; 10(4): 673–676.
-
38. Dahak K and Taourirte M.
Comparative Study of In Vitro Antimicrobial Activities of Foeniculum vulgare Mill. (umbelliferae) extract. OnLine Journal of Biological Sciences. 2013; 13(4): 115 - 120.
-
39. Hassan OA, Abu-Raghif AR, Rasheed AM, and Al-Yawer MA. Effect of Foeniculum vulgare Seed Aqueous Extract Eye Drops on Selenite induced Cataract in Rabbits. Int. J. Pharm. Sci. Rev. Res. 2017; 47(16);83-87.
-
40. Sadeghpour, Montaseri, Najafpour,
Dolatkhah, Rajabzadeh and Khaki. Study of Foeniculum vulgare (Fennel) seed extract effects on serum level of oxidative stress. Crescent Journal of Medical and Biological Sciences. 2015; 2(2): 59- 63.
-
41. Nassar MI, El–sayed, Makled, El– Khrisy and Osman. Secondary
metabolites and pharmacology of Foniculum vulgare Mill. Subsp. Piperitum . Rev latinoam. Quím. 2010; 38(2): 103-111.
-
42. Seevaratnam V., Banumathi P, Premalatha MR, Sundaram and Arumugam T. Functional Properties of Centella Asiatica (L.): A Review. International Journal of Pharmacy and Pharmaceutical Sciences. 2012; 4 (5); 8-14.
-
43. Prakash V. Terpenoids as Source of Anti-Inflammatory Compounds. Asian J Pharm Clin Res. 2017; 10(3):68-76.
-
44. Singh S, Gautam, Sharma and Batra. Centella asiatica (L.): A plant with immense medicinal potential but threatened. International Journal of Pharmaceutical Sciences Review and Research.2010; 4(2); 9-17
-
45. Kant R, Srivastav PP, and Datta AK. The Medicinal Role of Centella asiatica and Its Applications in the Dahi: A Research Review. Journal of Pharmaceutical Research International. 2019; 28(6): 1-9.
-
46. Roy DC, Barman, and Shaik. Current Updates on Centella asiatica: Phytochemistry, Pharmacology and Traditional Uses. Med Plant Res. 2013; 3(4):70-7.
-
47. Puttarak P. and Panichayupakaranant P. Factors Affecting The Content of Pentacyclic Triterpenes in Centella asiatica Raw Materials. Pharmaceutical Biology. 2012; 50 (12); 1508–1512.
-
48. Hashim P, Sidek H. Helme M, Helan M. Sabery A, Palanisamy UD, et al. Triterpene Composition and
Bioactivities of Centella asiatica. Molecules. 2011; 16; 1310-1322.
-
49. Chong NJ and Aziz. Systematic Review on Chemical Constituents of Centella asiatica, Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2011; 2(3); 445-459
-
50. Francis and Thomas. Essential Oil Profiling of Centella asiatica (L.) Urb-a
medicinally important herb. South Indian J Biol Sci. 2016; 2(1): 16973
-
51. Bhavana D and Jyoti. Centella asiatica: The elixir of life. International Journal of Research in Ayurveda and Pharmacy. 2011; 2(2): 431-438.
-
52. Sekar, Ayyanar, Pillai. Phytochemical Screening and Antibacterial Activity of Leaf and Callus Extracts of Centella asiatica. Bangladesh J Pharmacol. 2011. 6(1): 55-60.
-
53. Nasution MY, Martina R, Ahmad SS. Pulungan, Nanda P and Diky SD. Antimicrobial Activities of Centella asiatica Leaf and Root Extracts on Selected Pathogenic Micro-organisms. Journal of Medical Sciences. 2018; 18(4): 198-204
-
54. Dhiman, Aggarwal , Aneja, and Kaur. In Vitro Antimicrobial Activity of Spices and Medicinal Herbs Against Selected Microbes Associated with Juices. Int J Microbiol. 2016; 9015802: 1-9.
-
55. Idris NA, Nadzir MM. Antimicrobial Activity of Centella asiatica on Aspergillus niger and Bacillus subtilis. Chem Eng Trans. 2017; 56:1381-1386.
-
56. Sultan RA, Mahmood SB, Azhar I, Ahmed SW, Mahmood ZA. Biological Activities Assessment of Centella asiatica (Linn.). J Herbs Spices Med Plants. 2014; 20(3):319-327.
-
57. Chippada SC, Volluri SS, Bammidi SR, Vangalapati M. In Vitro Antiinflammatory Activity of Methanolic Extract of Centella asiatica by HRBC Membrane Stabilisation. Rasayan J Chem. 2011; 4(2):457-60.
-
58. Huang, S.S. et al. Antinociceptive Activities and the Mechanisms of AntiInflammation of Asiatic Acid in Mice. Evid Based Complement Alternat Med. 2011; 895857.
-
59. Wan J, Gong X, Jiang R, Zhang Z, and Zhang L. Antipyretic and AntiInflammatory Effects of Asiaticoside in Lipopolysaccharidetreated Rat Through Up-Regulation Of Heme oxygenase-1.
Phytotherapy Research. 2013; 27:
1136–1142.
-
60. Nhiem NX, Tai BH, Quang TH, Kiem PV, Minh CV, Nam NH, et al. New Ursane-Type Triterpenoid Glycoside From Centella asiatica Leaves Modulates the Production of Nitric Oxide and Secretion of TNF-α in activated RAW 264.7 cells. Bioorganic and Medicinal Chemistry Letters. 2011; 21 (6): 1777–1781.
-
61. Orhan IE, et al. Comparative studies on Turkish and Indian Centella asiatica (L.) Urban (gotu kola) samples for their enzyme inhibitory and antioxidant effects and phytochemical
characterization. Ind Crops Prod. 2013; 47: 316-322.
-
62. Chandrika UG, and Kumara PA. Gotu Kola (Centella asiatica): Nutritional properties and plausible health benefits. Adv Food Nutr Res. 2015; 76: 125-157.
-
63. Laribi K. Kouki, Hamdi M, and Bettaieb. Coriander (Coriandrum sativumL.) and its bioactive constituents. Fitoterapia. 2015; 103: 9– 26.
-
64. Zhang, Amila AD, Kudret K, and Muraleedharan GN. Evaluation of coriander spice as a functional food by using in vitro bioassays. Food Chem. 2015; 167: 24–29.
-
65. Khan, IU, Dubey W and Gupta V. Taxonomical Aspect of Coriander (Coriandrum sativum L.). International Journal of Current Research. 2014; 6(11); 9926-9930.
-
66. Yeung EC, and Bowra S. Embryo and endosperm development in coriander (Coriandrum sativum). Botany. 2011; 89: 263-73.
-
67. Mandal, S and Mandal M. Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine. 2015; 1-8.
-
68. Asgarpanah, J and N. Kazemivash. Phytochemistry, pharmacology and medicinal properties of Coriandrum
sativum L. African Journal of Pharmacy and Pharmacology. 2012 6(31);2340-2345.
-
69. Nadeem, Anjum FM, Khan MI, Tehseen S, El-Ghorab A, Sultan JI. Nutritional and medicinal aspects of coriander (Coriandrum sativum L.). Br Food J. 2013; 115: 743-55.
-
70. Anwar F, et al. Physicochemical composition of hydro-distilled essential oil from coriander (Coriandrum sativum L.) seeds cultivated in Pakistan. J. Medicinal Plants Research.2011; 5(15): 3537-3544
-
71. Sahib NG, Anwar F, Gilani AH, Hamid AA, Saani N, and Khalid MA.Coriander (Coriandrum sativumL.): A Potential Source of High-Value Components for Functional Foods and Nutraceuticals- A Review. Phytother Res. 2012; 27(10); 1439-1456.
-
72. Silva F, Ferreira S, Queiroz JA, Domingues FC. Coriander (Coriandrum sativum L.) essential oil: its
antibacterial activity and mode of action evaluated by flow cytometry. J. Med. Microbiol. 2011; 60(10):1479-1486.
-
73. Cao, Xin-Zin, You, Jian-Ming, Li, Shen-Xin, Zhang, and YouLiang. Antimicrobial Activity of the Extracts from Coriandrum sativum.Int. J. Food Nutrition and Safety. 2012; 1(2): 54-59.
-
74. Casetti FSB, Biehler K, Augustin M, Schempp CM, Frank U. Antimicrobial Activity Against Bacteria With Dermatological Relevance and Skin Tolerance of the Essential Oil From Coriandrum sativum L. Fruits. Phytother Res. 2012; 26(3):420-424.
-
75. Sambasivaraju D and Fazeel.
Evaluation of antibacterial activity of Coriandrum sativum (L.) against gram – positive and gram – negative bacteria. Int J Basic Clin Pharmacol. 2016; 5(6):2653-2656
-
76. Rahal A, Mahim VAK, Kumar A. Tiwari, R. Phytonutrients and nutraceuticals in vegetables and their multi-dimensional and health benefits
for humans and their companion animals: A review. J Biol Sci. 2014; 14: 1-19.
-
77. Oetari, R. A. Teknik Aseptis. Yogyakarta: Gadjah Mada University Press; 2018.
-
78. Depkes RI. Farmakope Indonesia. Edisi Keempat. Jakarta: Departemen
Kesehatan Republik Indonesia; 1995.
65
Discussion and feedback