Effect of Maternal Antibodies on Histopathogenesis of Newcastle Disease Virus in Broiler Chickens
on
Veterinary Science and Medicine Journal, February 2016
Vol 4 No 1: 27-31
Effect of Maternal Antibodies on Histopathogenesis of Newcastle Disease Virus in Broiler Chickens
I Made Galih Diparayoga1, Nyoman Mantik Astawa2 , Anak Agung Ayu Mirah Adi3 1Veterinarian in Bali Vet Clinics, Badung
2Laboratory of Veterinary Virology, Faculty of Veterinary Medicine, Udayana University, Denpasar , Bali , Indonesia
3 Laboratory of Veterinary Pathology, Faculty of Veterinary Medicine, Udayana University, Denpasar , Bali , Indonesia.
corresponding address : galihdiparayoga@gmail.com
ABSTRACT
The aims of this research were to overview the effect of maternal antibodies on the histopathological changes and viral antigen distribution of the broiler chickens challenged with ND APMV-1 virus. A total of 100 chicken were allotted into 3 treatment groups consisting of group I (titer antibodies< 23 HI Unit), group II (titer antibodies 23 – 24 HI Unit) and group III (titer antibodies> 24 HI Unit). All group I, II and III were inoculated with ND virus isolates of type viscerotropic velogenic at the dose of 1000 TCID50. The histopathological changes observed in nervous system were endotheliosis and perivascular cuffing. Immunohistochemistry staining showed that NDV infected cells were found in most organs both in inflammatory cells and in epithelial cell of many organs mainly in nervous, respiratory and digestive systems. Neurological symptoms and neural lesions were highest in group II (titer antibodies 23 – 24 HI Unit).
Key words: maternal antibodies, newcastle disease, histopathological, immunohistochemistry
INTRODUCTION
Avulavirus genus belongs to the family of Paramyxoviridae and is designated as avian paramyxovirus type 1 (APMV-1), one of 12 identified APMV serotypes (Alexander, 2000). Serological tests are useful tools in diagnosis of infection. Hemaglutination inhibition (HI) test is the most common test used for detection of immune response in affected birds (Alexander and Senne, 2008b). Newcastle Disease (ND) causes huge economic losses to the commercial poultry farmers around the world (Qin et al., 2008). Etiological agents of ND are virulent strains of avian paramyxovirus-1 (Yu et al., 2001). The disease is characterized by respiratory, nervous system impairment, gastrointestinal and reproductive problems (Alexander and Senne, 2008).Tabbu (2000) reported that chicks from immunized parents had high level of maternally derived antibodies (MDA) which protect them from virulent and vaccine viruses. It was also reported that MDA are protective and neutralize vaccine virus if the
chicks are vaccinated in the presence of high level of MDA. In order to formulate appropriate vaccination schedule and control measures, the serological status of NDV among chickens need to be elucidated. The vast majority of bird species appear to be susceptible to infection with APMV-1 of both high and low virulence for chickens, although the clinical signs seen in infected birds vary widely and are dependent on factors such as: the virus, host species, age of host, infection with other organisms, environmental stress and immune status (Kencana, 2012).
The titer of MDA naturally has an effect on the pathogenesis of ND in chickens especially on the occurrence of neurological symptoms of ND.. The subprotective levels of MDA which cannot prevent chickens from NDV infection but prevent from death, often causes prolonged chronic infection. Such prolonged chronic infection often cause the spread of the virus into the brain. As a result, neuronal clinical signs are often observed with permanent brain damages. A study was then
conducted to investigate the effect of maternal antibodies in serum on the occurrence of neuronal signs in chickens challenged with virulent strain of NDV.
MATERIALS AND METHODS
This was an experimental study with randomized design. A total of 100 chickens was allotted into 3 treatment groups according to their maternal antibodies titer against NDV: group I (titer antibodies< 23 HI Unit), group II (titer antibodies 23 – 24 HI Unit) and group III (titer antibodies> 24 HI Unit). Group I, II and III were challenged with velogenic ND virus at 1000 TCID50 per chicken. Two weeks post infection, chickens were necropsied and specimens from each organ were prepared for histopathological and immunohistochemical staining.
One ml blood was collected from the brachial vein and was left overnight at 4oC. Serum was separated from blood clot by centrifugation at 2000 rpm for 5 minutes. Serum was then aliquoted in eppendorf tubes and stored at -20oC.
HI test was perfomed to determine the maternal antibodies titer in each chicken. Serial two-fold dilution of serum from each chicken was prepared. Four HA units NDV were then added into each serum dilution and incubated for 30 minutes at room temperature. Suspension of 1 % chicken red blood cells was added, shaked and incubated for 15 minutes at room temperature. The antibodies titer was determined as antilog of the highest dilution of serum which was capable to completely inhibit virus to hemaglutinate red blood cells and was expressed as HI units.
On the age of 12 days, chickens were grouped according to their antibody titer and all chickens were challenged with 1000 TCID50 the velogenic NDV virus that had previously been titrated in chicken embryo fibroblast cell culture. The challenge virus was firstly titrated in chicken embryo fibroblast cell culture. The virus was diluted to obtain 1000 TCID50 per 0.1 ml. The challenge procedure was conducted by inoculation of 1000 TCID50 intraocularly into
both eyes. The challenged chickens were observed for the presence of ND clinical signs and mortality. Chickens died with clinical signs of ND were necropzied for histophatological and immunochemistry examinations. Chickens that survived for 2 weeks following the challenge test, either with or without ND clinical signs were also sacrificed for histopathological and immunochemistry examination.
Histopathological and Immunochemistry Examination.
Organs from all sacrificed chickens were subjected for tissue processing to prepare paraffin embedded tissue according to routine procedures. The tissue in paraffin block were the cut at 4 micron thin and was layered on poly-L-lysine coated microscope slides. For histopathological examination, the tissues were stained with hematoxyline and eosin (HE) according to the routine procedures. For immunohistochemistry staining, tissues on microscope slides were firstly deparaffinized with xylene (2 x 5 minutes), rehydrated with absolute ethanol (2 x 5 minutes), and washed with PBS (2 x 2 minutes). Antigen retrieval was conducted by heating the tissues at 95oC for 20 minutes in citrate buffer pH 6.0. Endogenous peroxidase was inactivated by treated tissues with 3% H2O2 for 20 minutes at room temperature. After twice washed with PBS, 100 μl monoclonal antibodies against NDV (diluted 1:20 in PBS with 2% skim milk was added and incubated for overnight at 4oC. Anti-mouse IgG conjugated with biotin was then added (Biocare USA) and incubated for 30 minutes at room temperature. Streptavidin-HRP (Biocare) was then added and incubated for 20 minutes at room temperature. Following extensive washes (4 x 5 minutes) with PBS, diazinobenzidine (DAB) substrate was then added for 5 minutes at room temperature. Cells in tissues were counterstained with Mayer hematoxyline and dehydrated with ethanol (2 x 5 minutes), cleared with xylene (2 x 5 minutes) and mounted with entellan. The cells bearing NDV antigen were observed on light microscope.
RESULTS AND DISCUSSION
Incubation Period and the Duration of Clinical Signs Post-Challenge
Following the challenge test with 0.1 ml of 1000 TCID50 by intraocular inoculation on the left and right eyes, various clinical signs were observed. The incubation period was 1-6 days with an average of 3.33 days. The duration of illness was 1-9 days with an average of 4.76 days.
Effect of Maternal Antibodies Titers on the Incubation Period, Survival and Clinical Signs
In chickens with maternal antibodies titer of <23, the average incubation period was 3.47
days and the duration of illness were 4.36 days. In chickens with maternal antibodies titer 23-24, average incubation period and duration of illness was 8.1 days and 5.7 days respectively. Meanwhile, in chickens with maternal antibodies titer of 25, the average incubation period and duration of illness were 6 days and 8 days respectively.
Histopathological Changes of Brain, Lung and Intestine
Histopathological changes of brain in 3 treatment groups of chickens were perivascular cuffing with different levels. The most extensive perivascular cuffing was found in the brain of chickens with titer antibodies 23-24 (Figure 1 and 2).
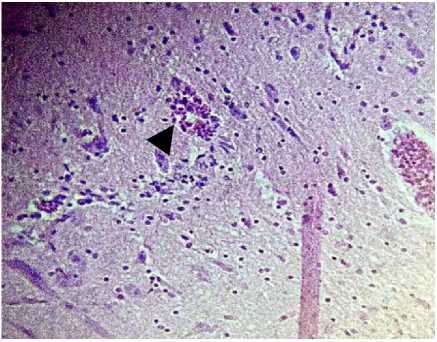
Figure 1. Found Endotheliosis, perivascular cuffing and edema perivascular (arrow) magnification 400 X (Titer AB 23 – 24)
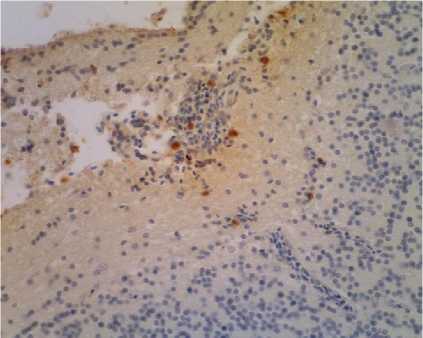
Figure 2. Positive cells containing viral antigen found in inflammatory cells ND (arrow) magnification 400 X (Titer AB 23 – 24)
Effect of Maternal Antibodies Titer on the Distribution of Virus In Brain, Lungs and Intestines
In the brain of chickens with maternal antibodies titer of 23-24 HI units, the average degree of ND virus infection was 3.86. This was significantly higher than those in chickens with maternal antibodies titer of <23 (2.48) and > 25(1.50). In the intestinal organs, the degree of infection were highest in the group of chickens with maternal antibodies titer <23 (2.86) and the lowest degrees of infection was
noted in the group of chickens with maternal antibodies titer > 25 (1.00).
Role Maternal Antibodies in Clinical Signs of ND
At 12 days old chicks when the challenge test was conducted, the titer of maternal antibodies against ND virus in chickens was mostly bellow the protective level. The protective level of antibodies against ND minimum was reported 25 HI Units (Suryana, 2006). However, several chickens still had
antibodies titer above the protective level (25 HI units). Chickens with maternal antibodies titer of <23 HI units generally died with typical clinical signs of ND post challenge. Protective antibodies titers against ND virus in chickens infection was > 24 HI Unit. Suryana (2006) reported that chickens with average antibodies titer minimum 25 HI Unit had 100% protection rate, chickens with maternal antibodies titer of > 24 HI units had 60% protection rate, and those with maternal antibodies titer of > 23 HI units had 40% protection. While in the control group of unvaccinated chickens, protection rate was 0%. Kusmaedi (2001) and Allan (1978) stated that challenged test ND, chickens contained antibodies <22 HI Unit resulted 100% died. Antibodies titers between 22-24 units mortality rate reached 10%, but antibody titer 25-26 HI Unit resulted in 0% mortality.
Table 1. Average Score Immunohistochemistry
ND Virus Antigen in Organs of the Brain, Lungs and Intestines
|
Group of maternal antibodies titers |
Brain |
Lungs |
Intestines |
|
Group 1 (< 23 HI Unit) |
2.48 |
2.76 |
2.86 |
|
Group II (23-24 HI Unit) |
3.86 |
2.43 |
2.00 |
|
Group III (> 24HI Unit) |
1.50 |
2.00 |
1.00 |
The Effect of Maternal Antibodies Titer on the Degree of Viral Infection in Brain and Other Organ.
Velogenic ND virus in Indonesia has been viscerotropic or velogenic viscerotropic (Adi et al., 2010). One phenomenon attempted to disclose in this study was the appearance of neurological symptoms in chickens infected with ND virus. The results of immunohistochemistry staining of the brain tissue showed that chickens with maternal antibodies titer of 23-24 HI units had the highest score of ND virus infection as compared to those in chickens with maternal antibodies titer of 25 HI units and <23 HI units. The results of histopathological changes indicated that chickens infected with neurological symptoms also suffered endotheliosis, perivascular cuffing and edema perivascular in the brain. These
results were similar to that reported by Bhaiyat et al., (1994) that perivascular cuffing in acute infection, lymphocytes capillary and venous edema had been associated with ND virus infection process. Newcastle diseases virus neurological damages infected were found by (Ecco et al., 2011). Infected ND virus did not damage the neurological cells but only damage in vascular endothelial marked hypertrophy, vasculitis specifically cerebellum (Nakamura et al., 2008).
Distribution ND virus in nervous organ reported by Ecco et al. (2011) found in
chickens infected with isolated velogenic imunopositive viscerotropic and reaction ND virus would be inflammatory cells and astrogliosis. However, the infection of NDV in chickens could resulted in neurological symptoms which persist for prolonged period after infection. The role of subprotective antibodies titers had been suggested from several studies. When antibodies titer in chickens against ND virus is at subprotective level, it cannot prevent infection, but can protect chickens from death following infection. This study demonstrated that the level of meternal antibodies in chicks played important ruler in protecting against infectious ND virus infection. The titer of > 24HI Unit, seemed to give the best protection, therefore vaccination program in productive poultry should be evaluated regularly.
REFERENCES
Adi AAA, Astawa NM, Putra NM, Hayashi KSA, Matsumoto Y. 2010. Isolation and characterization of a pathogenic Newcastle disease virus from a natural case in Indonesia. J Vet Med Sci. 72(3):313-319.
Alexander DJ. 2000. Newcastle disease and other avian paramyxoviruses. JRev Sci Techs. 19(2): 443-462.
Alexander DJ, Senne DA. 2008. Newcastle disease, other avian paramyxoviruses, and pneumovirus infections. In Diseases of Poultry, J Gen Virol. 80(2):75–116.
Alexander DJ, Senne DA. 2008b. Newcastle disease and other avian pramyxoviruses. In: A Laboratory manual for the isolation, identification and characterization of
avian pathogens, Dufour-Zavala L. American Association of Avian Pathologists. 7(2): 135–141.
Allan WH, Lancaster JE, Toth B. 1978. Newcastle disease vaccines-their production and use. FAO animal production and health series, 25(10): 113.
Bhaiyat MI, Ochiai K, Itakura C, Islam MA, Kida H. 1994. Brain lesions in young broiler chickens naturally infected with a mesogenic strain of Newcastle disease virus. Avian Pathol. 23(4):693-708.
Ecco R, Susta L, Afonso CL, Miller PJ, Brown C. 2011. Neurological lesions in chickens experimentally infected with virulent Newcastle disease virus isolates. Avian Pathol. 40(2):145-152.
Kencana GAY. 2012. Penyakit Virus Unggas. Udayana University Press. Denpasar.
Kusmaedi. 2001. Teknik Uji Hemaglutination Inhibition Untuk Mengukur Tingkat Kekebalan Terhadap Newcastle: 224-232.
Nakamura K, Ohta Y, Abe Y, Imai K, Yamada. 2010. Pathogenesis of
conjunctivitis caused by Newcastle disease viruses in specific-pathogen-free chickens, Avian Pathol. 33(3):371-376.
Qin ZM, Tan LT, Xu HY, Ma B,. Wang YL, Yuan XY, Liu WJ. 2008. Pathotypical characterization and molecular
epidemiology of Newcastle disease virus isolates from different hosts in China from 1996 to 2005. J. Clin. Microbiol. 46(2):601-611.
Suryana N. 2006. Pengamatan daya proteksi ayam post vaksinasi newcastle disease dengan uji tantang. Temu Teknis Nasional Tenaga Fungsional Pertanian. Pusat Penelitian dan Pengembangan Peternakan. 21(37) :35 - 39
Tabbu CR. 2000. Penyakit Ayam dan Penanggulangannya: Penyakit Bakterial, Mikal, dan Viral. Kanisius, Yogyakarta.
Yu L, Wang Z, Jiang Y, Chang L, Kwang J. 2001. Characterization of newly emerging Newcastle disease virus isolates from the People’s Republic of China and Taiwan. J. Clin. Microbiol. 39(10): 3512 - 3519.
31
Discussion and feedback