The Effect of Soyabean Extender on Viability and DNA Integrity of Kintamani Dog Sperm on Cold Storage
on
Veterinary Science and Medicine Journal, February 2016
Vol 4 No 1: 11-16
The Effect of Soyabean Extender on Viability and DNA Integrity of Kintamani Dog Sperm on Cold Storage
I Made Yoga Windu Pradana1*, Wayan Bebas2, I Ketut Puja 3 1Veterinary Clinics, tudent, Udayana University, Bali 2Laboratory of Veterinary Reproduction, Faculty of Veterinary Medicine, Udayana University, Bali
3Veterinary Genetics and Reproduction Technology Laboratory, Faculty of Veterinary Medicine, Udayana University, Bali
*Corresponding author: vindu_vet@yahoo.com
ABSTRACT
The objective of this study was to evaluate the viability and integrity of canine semen diluted in egg yolk glucose-citrate and Tris-citrate-glucose-soya bean. Semen was collected by manual manipulation from an apparently healthy kintamani dog stud. The semen was subjected to gross and microscopic examination to determine its viability following dilution in different extenders. Only semen with motility rate of 60% or higher was used in this study. Semen samples were diluted in Tris-citrate-glucose-soya bean and egg yolk glucosecitrate extenders at two levels of dilution; sperm to extenders ratio of 1:2 and 1:3. Following semen dilution, evaluations were made on sperm motility, percentage of live sperm, and DNA integrity at 0 hour, 3 hours, and 6 hours under 5oC of storage. Data was analyzed by multivariate analysis of variance (MANOVA).The present results showed a high significant different (p<0.01) in sperm motility, percentage of live and DNA integrity between treatment with egg yolk glucose-citrate and Tris-citrate-glucose-soya bean. The result also showed that percentage of live sperm and DNA integrity of semen at 1:2 dilution difference significantly (p<0.01) to that of 1:3 dilution. The sperm viability and integrity were significantly difference (p<0,01) between the duration of storage at 5°C. Thus, the current study indicated that Tris-citrate-glucose- soya bean extenders was sufficient to maintain motility, viability, and DNA integrity of kintamani dog spermatozoa during storage at 5 oC. Further research should be conducted to evaluate the fertility of spermatozoa following dilution with these extenders.
Keywords: viability, DNA integrity, semen, kintamani dog
INTRODUCTION
Kintamani dog is recently considered as a new breed of dog from Indonesia. It is a local dog of mountaineus area around the village of Sukawana, Kintamani, Bangli, Bali. At present days, dog keeper at Kintamani start to breed it conventionally usually by bringing a superior males to mate female in estrus. This conventional way of mating may lead to various impacts and problems due to transport of live animals over long distances (Gunawan, 2013). One of the possible solutions is by conducting artificial insemination (AI) instead of natural mating. Artificial insemination offers the opportunity to overcome the limitations of time and space as well as allowing breeders to mate dogs that may be disadvantageous in a natural environment. Artificial insemination using chilled dog semen have nowadays
become very popular among dog breeders and practiced world widely (Rijsselaere et al., 2011).
The successful artificial insemination in dogs have been reported using fresh and cold semen either (Tsutsui et al., 2003) and frozen semen (Hayashi et al., 2013). Artificial insemination with chilled and frozen-thawed semen gained increasing interest amongst dog breeders, veterinarians and experimental research facilities worldwide (Rijsselaere et al., 2011).
For the purpose of AI, semen should be diluted in proper extenders. Egg yolk is the most commonly component in canine semen extenders to protection sperm from cold shock and disruption during freezing and thawing process. However, several countries have, in fact, banned export and import of canine frozen
semen that contains egg yolk due to the recent outbreak of avian influenza (Abe et al., 2008). Thus, it may an important effort to develop alternative semen extenders without egg yolk for use in chilling and freezing of canine sperm. As an alternative replacement to egg yolk, soya bean extract seems to be suitable as a semen extender for canine species, due to its proportion related with the same function with egg yolk (Aires et al., 2003). Therefore, the aim of this study was to evaluate the ability of soya bean extender applied at different concentration for sperm dilution of kintamani dog.
MATERIALS AND METHODS
Animal Experiments
The present study used four kintamani stud dogs obtained from Asubali Kennels; they were clinically showed no abnormalities and at the age of 1.5 to 4 years. The dogs were maintained in individual outdor pens in kennel and feed dryfood twice daily and had free access to water.
Semen Collection and Evaluation
Semen was collected using manual stimulation method. Total 36 ejaculates were collected for evaluation .The sperm-rich fraction of each ejaculate was macroscopically evaluated, and the volume was measured. Sperm motility and morphology were evaluated using a light microscope (100x) according to the procedures of Puja (2015). Sperm concentration was determined with a Neubauer counting chamber (Johnston et al., 2001). Percentage of live spermatozoa was evaluated by staining with Eosin Nigrosin stain and DNA damage was evaluated by staining with Acridine Orange (AO) stain (Tejada et al., 1984). Only samples with sperm concentration of >200 x 106spermatozoa/ml and sperm motility >80% were used in this research.
Sperm Dilution.
Each sperm collected from four studs was divided into two aliquots. One aliquot was diluted with extender based on soya bean
extract consisting of 25% soya bean extract, tris (3.8 g), citric acid (2.2 g), and glucose (0.6 g). The other aliquot was diluted with citrate-egg yolk-glucose extender prepared according to Moss et al. (2000). The sperms were diluted at concentration ratio of sperm and extender of 1:2 and 1:3. They were then evaluated at 0 hours, 3 hours, and 6 hours at 5oC of storage.
Statistical Analysis
Data regarding the viability of sperm and DNA integrity were analyzed by multivariate analysis of variance (MANOVA) using SPSS ver.17 for window and if there are significant differences between treatments, the analysis was continued with LSD (Heath, 2001).
RESULTS AND DISCUSSION
The kintamani dogs in the current study were noted to have good quality of raw semen. Semen colour was creamy white and their volume 1–2.5 ml. The percentage of sperm motility, viability, and DNA integrity were 90%, 98%, and 95%, respectively. The sperm concentration was 550x106 spermatozoa/ml.
The result for motility rate following sperm dilution at different dilution ratio and at different storage time in a temperature of 5°C is shown in Table 1. Table 1 shows that the motility rate was significantly decreased following the time of storage on both types of diluents, and either at a dilution ratio of 1:2 or 1:3 (p<0.01). However, the percentage of motility after storage until 6 hours was still above 60%. At dilution ratio of 1:2, dilution with egg yolk glucose-citrate extenders led to sperm motility rate of 78.67%±3.61, whereas with tris-citrate-glucose-soya bean extender was 74.17%± 4.91. In this study, the dilution level of 1:2 resulted in the highest percentage of motility of the spermatozoa.
The average percentage of live spermatozoa at different dilution ratios and different storage time in a temperature of 5°C is shown in Table 2. Although percentage of live of spermatozoa gradually decreased during chilling, the viability of sperm from both extenders were still high (> 60%).
Table 1. The Average Motility Rate of Spermatozoa Following Sperm Dilution with Different
Extenders, and Different Dilution Ratio and Duration of Storage at Temperature of 5°C
|
Examination |
Diluent |
Ratio of diluents |
Duration of storage | ||
|
0 hour |
3 hours |
6 hours | |||
|
Egg yolk |
1:2 |
84.00±4.19 |
81.67±4.41 |
78.67±3.61 | |
|
Motility Rate (%) |
glucosecitrate |
1:3 |
80.33±5.27 |
78.50±5.08 |
76.17±4.79 |
|
Tris-citrate- |
1:2 |
79.83±4.53 |
76.50±4.63 |
74.17±4.91 | |
|
glucose-soya bean |
1:3 |
78.17±4.44 |
76.17±3.43 |
73.83±3.48 | |
The average percentage of live spermatozoa at different dilution ratios and different storage time in a temperature of 5°C is shown in Table 2. Although percentage of
live of spermatozoa gradually decreased during chilling, the viability of sperm from both extenders were still high (> 60%).
Table 2 The Average Percentage of Live Sperm Following Dilution at Different Extenders, Dilution Ratios and Storage Times at Temperature of 5°C
|
Examination |
Diluent |
Dilution ratio |
Storage Time | ||
|
0 hour |
3 hour |
6 hour | |||
|
Egg yolk |
1:2 |
94.67±2.25 |
92.67±2.16 |
90.50±1.87 | |
|
glucose-Citrate |
1:3 |
92.33±2.42 |
89.83±1.47 |
87.33±2.42 | |
|
Viability | |||||
|
rate (%) |
Tris-citrate- | ||||
|
glucose- soya |
1:2 |
93.17±2.22 |
90.67±2.80 |
88.50±2.42 | |
|
bean |
1:3 |
90.00±3.09 |
87.33±3.67 |
85.17±3.37 | |
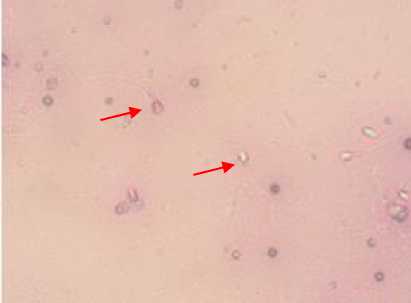
Figure 1. Dead spermatozoa (A) and live spermatozoa (B) after stained with eosin and negrosin stain.
The average percentage of live sperm (see Figure 1) in citrate-glucose-egg yolk diluent at 6 hours of storage was 90.50%±1.87 and in tris-citrate-glucose-soya bean diluent was 88.50%±2.42. In this study, the level of dilution of 1:2 have resulted in the higher percentage of live sperm. Significant effect (p<0.01) on the percentage of live sperm was noted due to different diluents, dilution ratio, and storage times. However, interaction between diluent and dilution ratio and between the ratio of diluent and storage time showed no significant different (p>0.05).
The sperm DNA integrity in is shown in Table 3. The sperm DNA integrity was not significantly different between dilution with
tris-citrate-glucose-soy bean extract ex and egg yolk glucose-citrate extenders. Following storage time of 6 hours, it can be noted that the rate of DNA damage DNA was 10-16%. Thus, the use of tris-citrate-glucose-soya bean extract and egg yolk glucose-citrate extenders may
preserved DNA integrity well at 5o C of storage up to 6 hours. The characteristics of damaged DNA of kintamani dog sperm can be seen from Figure 2 in which acridine orange staining was performed. When DNA is damaged, the spermatozoa will be bright green in color.
Table 3. Average Damage of Sperm DNA Due to Dilution with Different Extenders, Dilution Ratios and Storage Time at Temperature of 5°C.
|
Examination |
Diluents |
Dilution ratio |
Storage Time | ||
|
0 hour |
3 hours |
6 hours | |||
|
Egg yolk |
1:2 |
94.67±1.36 |
92.50±1.04 |
90.33±1.36 | |
|
glucose-citrate |
1:3 |
90.00±0.89 |
86.67±1.03 |
84.33±0.81 | |
|
DNA | |||||
|
Integrity |
Tris-citrate- | ||||
|
glucose- soya |
1:2 |
95.33±1.21 |
93.17±0.98 |
90.50±1.04 | |
|
bean |
1:3 |
90.83±1.16 |
88.83±1.16 |
87.17±0.98 | |
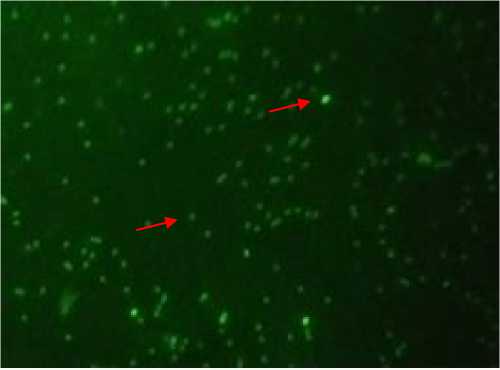
Figure 2. Start denatured (A) and normal DNA (B) stained with acridine orange stain
DISCUSSION
Extenders used for semen dilution should provide suitable media for survival of spermatozoa for some times. Egg yolk-based extenders are commonly used in chilled semen. Some research findings have shown that citrate-yolk-glucose diluents were able to effectively preserve motility and viability of spermatozoa (Hori et al. 2014). Egg yolk contains source of energy, a kind of buffer and may provide suitable osmotic pressure. It had high protein and carbohydrates in the form of glucose,
galactose, and polysaccharides. Carbohydrates as large molecules can provided energy in considerable amounts required for normal metabolism and physiological process (Naing et al., 2010). Thus, as shown in the current study, the presence of soya bean in extender may equally effective in preserving the motility and viability of kintamani dog sperm stored at 5ºC until 6 hours compared to egg-yolk extender. Previous studies demonstrated that soya bean extenders were effective for diluting ram sperm (de Paz et al., 2010), enhanced the improvement of sperm parameter (Zhang et al., 2009), and
soya lecithin-based extenders were better when compared to milk, tris-citric egg yolk and egg yolk-citrate extenders of buffalo sperms stored at 5ºC (Akhter et al., 2011).
The result in this study shows that the motility and percentage of live spermatozoa was significantly decreased following the time of storage on both types of diluents examined. However, the motility can be considered as still high. This was obvious after storage of semen at 5ºC for 6 hours; the motility can be effectively preserved. Storage of semen in low temperatures (5°C) can damage the sperm as a result of cold shock. Egg yolk contains large amount of lipoprotein which help in protecting the membrane damage (Neves et al., 2014). Also soya bean contains large amount of lipoprotein called soya lecithin which has similar properties to egg yolk lecithin in protecting membrane against cold shock (Rehman et al., 2014). Although the percentage of motility decreased until the 6 hour of storage, the motility rate (>60%) can be considered as still suitable for the purpose of artificial insemination of semen. According to Johnston (2001), the minimum sperm motility that can be used for artificial insemintion in dogs was 60%.
Besides motility and the percentage of live spermatozoa, the percentage of DNA integrity is another factor that determine the quality of spermatozoa. Agarwal and Said (2003) reported that the successful fertilization is influenced by the integrity of the sperm DNA which was one of the parameters to be assessed in determining the quality of spermatozoa. Based on results of the present study, it can be noted that the tris-citrate-glucose-soya bean extender was effective in preserving spermatozoa DNA integrity during storage. This due to the fact that soya bean contains isoflavones (Astuti, 2008). Isoflavone is a type of antioxidant that has a potency to neutralize free radicals and reduce Reactive Oxygen Species (ROS). Free radicals and ROS compounds can promote lipid oxidation, protein oxidation, DNA strand breaks, DNA-based modifications, and modulation of gene expression (Lee et al., 2004).
In the current study, citrate-glucose-egg yolk and tris-citrate-glucose-soya bean has been noted are both able to protect the DNA integrity. The types of extender used had not significantly affected the percentage of spermatozoa with damaged DNA after storage up to 6 hours. In this study, the spermatozoa did not undergo full denaturation. Spermatozoa with full denaturation will be red in color when the sperm is examined under the fluorescence microscope (Tejada et al., 1984). However, spermatozoa with yellow-green fluorescent color means that it start to undergo denaturation. Differences in intensity of the color produced depends on the condition of condentation of chromatin of the spermatozoa. The Acridine Orange (AO) test is used to demonstrate structural modifications such as DNA denaturation, which may occur in the cell nuclei (Bencharif et al., 2013).
CONCLUSION
The current study indicated that tris-citrate-glucose-soya bean extenders may be sufficient to provide protection on the maintenance of motility, viability and DNA integrity of kintamani dog spermatozoa during storage at 5oC. Further research should be conducted to evaluate fertility of the spermatozoa following dilution with these extenders.
REFERENCES
Abe Y, Lee DS, Sano H. 2008. Artificial insemination with canine spermatozoa frozen in a skim milk/glucose-based extender. J Reprod Dev. 54:290-294.
Agarwal, and Said A. 2003. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum. Reprod. Update. 9:331-345.
Aires VA, Hinsch KD, Schloesser FM, Bogner K, Schloesser SM, Hinsch H. 2003. In vitro and in vivo comparison of egg yolk-based and soybean lecithin based extenders for cryopreservation of bovine semen. Theriogenology 60(2): 269-279.
Akhter S, Ansari MS, Rakha BA, Ullah N, Andrabi SMH, Khalid M. 2011. In vitro evaluation of liquid-stored buffalo semen at 50C diluted in soya lecithin based extender (Bioxcell), tris-citric egg yolk, skim milk and egg yolk-citrate extenders. Reprod. Dom. Anim. 46: 45–49.
Astuti S. 2008. Isoflavon kedelai dan potensinya sebagai penangkap radikal bebas. Jurnal Teknologi & Industri Hasil Pertanian 13(2) : 126-136.
Bencharif D, Amirat-Briand L, Le Guillou J, Vialtelle C, Anton M, Schmitt E, Desherces S, Barriere P, Tainturier D. 2013. Refrigeration of canine sperm at + 4°C: Comparative study of four different extenders for the refrigeration of canine sperm at +4°C: LDL, Tris egg yolk, Equex®, and INRA96®. Revue Med. Vet. 164 (5): 252-262.
de Paz P, Esteso MC, Alvarez M, Mata M, Chamorro CA, Anel L. 2010. Development of extender based on soybean lecithin for its application in liquid ram semen. Theriogenology. 74: 663– 671.
Gunawan NF. 2013. Viabilitas dan Integritas DNA Spermatozoa Anjing Kintamani pada Pengencer Air Kelapa Muda (Cocos nucifera). Thesis Megister Kedokteran Hewan Pasca Sarjana Universitas Udayana, Bali.
Hayashi K, Morita R, Aso T, Ono M, Ohtaki T, Tanemura K, Watari T, Tsumagari S. 2013. Evaluation of transcervical insemination using frozen semen by flexible endoscope in dog. J. Vet.Med. Sci. 75:315-318.
Heath D. 2001. An Introduction to Experimental Design and Statistics for Biology. UCL Press. London.
Hori T, Yoshikuni R, Kobayashi M, Kawakami E. 2014. Effects of storage temperature and semen extender on stored canine semen. J. Vet. Med. Sci. 76(2): 259–263
Johnston SD, Root MV, Olson PN. 2001. Semen Collection, Evaluation, and Preservation. In: Jhonston SD, Root Kustritz MV, Olson PNS, editors. Canine and Feline Theriogenology. Philadelpia: WB Saunders Co;2001:287.
Lee J, Koo N, Min DB. 2004. Reactive oxygen species, aging, and antioxidative
nutraceuticals.. Compre Rev. in Food Sci. and Food Safety. 3: 21-33.
Moss JA, Melrose DR, Reed HC, Vandeplassche M. 2000. Spermatozoa, Semen and Artificial Insemination In: fertility and infertility in domestic animals. Laing JA. London: Bailliere Tindal; 1979; 43-45.
Naing SW, Wahid H, Azam MK, Rosnina Y, Zuki AB, Kazhal S, Bukar MM, Thein M, Kyaw T, San MM. 2010. Effect of sugars on characteristic of boer goat semen after cryopreservation. Anim Reprod Sci. 122 : 2328.
Neves MM, Heneine LGD, Henry. 2014. Cryoprotection effectiveness of low concentrations of natural and lyophilized LDL (low density lipoproteins) on canine spermatozoa, Arq. Bras. Med. Vet. Zootec. 66 (3):769-777.
Puja IK. 2015. Aspek Reproduksi Pada Pengembangbiakan Anjing. Udayana
University Press: Denpasar.
Rehman FU, Qureshi MS, Khan RU. 2014. Effect of soyabean extenders on sperm parameters of holstein-friesian bull during liquid storage at 4oC. Pakistan J. Zool. 46(1) : 185-189.
Rijsselaere T, Maes D, Van den Berghe F, Van Soom A. 2011. Preservation and shipment of chilled and cryopreserved dog semen. Vlaams Diergeneeskundig Tijdschrift. 80 :248-253.
Tejada RI, Mitchell JC, Norman A, Marik JJ, Friedman S. 1984. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil Steril. 42:87–91.
Tsutsui T, Tezuka T, Mikasa Y, Sugisawa H, Kirihara N, Hori T, Kawakami E. 2003. Artificial insemination with canine semen stored at a low temperature. J. Vet. Med. Sci. 65(3) : 307 -312.
Zhang SS, Hu JH, Li WQ, Jiang ZL, Xiaoying Z. 2009. The croyoprotective effects of soybean lecithin on boar spermatozoa quality. Afr. J. Biotechnol. 8: 6476-6480.
16
Discussion and feedback