The Extraction and Phytochemical Test of Toxic Extract of Xestospongia testudinaria Sponge
on
Journal of Health Sciences and Medicine, Vol. 2 No. 1, February 2018
Extraction and Phytochemical Test of Toxic Extract of Xestospongia testudinaria Sponge
I Made Dira Swantara1, Wiwik Susanah Rita2
1Applied Chemistry, Graduate School
Udayana University
Jalan P. B. Sudirman, Denpasar, Bali, Indonesia
2Department of Chemistry, Faculty of Mathematics and Natural Sciences
Udayana University
Bukit Jimbaran, Badung, Bali, Indonesia
Abstract This research aims toextraction and phytochemical test of the toxic extract of the Xestospongia testudinaria sponge collected from Sanur beach, Bali, Indonesia. Extraction of the sponges was carried out by methanol at room temperature. Toxicity screening test was done based on Bhrine Shrimp Lethality Test (BSLT). The compounds of the toxic extract were performed by phytochemical test. Based on the results, it was found that the methanol extract of X. testudinaria sponges has toxic activity with LC50 of 31.62 ppm. The toxic extract contained alkaloid, steroid, polyphenol, and saponin compounds.
Keyword: phytochemical test, toxic extract, Xestospongia testudinaria sponge
marine natural ingredients and provided a variety of new cancer-fighting alternatives [2].
Sponges are one of the many biota in the sea. In Indonesian waters it is estimated that there are more than 1,000 species (types) of sponges. Reportedly sponge is a highly prospective bioactive material from the ocean. Nearly 5,000 compounds have been successfully isolated from these animals with activities such as antimicrobials, antifungals, antivirals, and anticancer drugs [2]. Sponges are potential marine organisms for exploration of new anticancer compounds because sponges produce the antiviral and cytotoxic compounds [3]. One of the sponges found on the coast of Sanur, Bali is Xestospongia testudinaria (X. testudinaria). Various studies have been conducted to isolate the compounds of the type spoge X. testudinaria. Quinn and Tucker [4] isolated brominated bis-acetylenic acetic acid. Further more Quinn and Tucker [5] also isolated two new compounds of brominated acetylenic acids. Pham et al. [6] isolated brominated acetylenic fatty acid and two esterified sterol. Jiang et al. [7] isolated four brominated aliphatic hydrocarbon and sterol. Lee et al. [8] isolated Marinobacter xestospongiae sp. Nov. Sun et al.[9] isolated the new compound bisabolane sesquiterpenoid. Nguyen et al. [10]. reported isolating anti-fouling compound 26,27-cyclosterol. Toxicity of X. testudinaria has been reported by Zhou et al. [11]. According to their report, the five compounds contained in X. testudinaria
(Sapinofuranone; Xestospongic acid; 24-hydroperoxy-24-vinyl-cholesterol; Saringosterol; and 29-hydroperoxystigmasta-5,24-dien-3β-ol) were toxic towards larvae of A. Salina with LC50 values varies between 0.56 and 6.99 µM.
Prescreening test of a substance potentially having anticancer activity is by toxicity test using Bhrine Shrimp Lethality Test (BSLT) method. Concentration sample with the 50% of mortality (LC50) is determined using bioindicator of A. salina larvae. If a substance has a toxicity (LC50) lower than 1,000 ppm, then the material has the potential to have anticancer activity, then it can be tested for cancer cells [12].
-
II. METHODS AND PROCEDURES
-
A. Materials
Sponges X. testudinaria was collected from Sanur beach, Bali, Indonesia, on April 25th, 2017. The sample was cleaned of impurities with tap water until clean, then it dried at room temperature for several days. After drying, subsequently it ground to the degree of fineness of 100 mesh.
-
B. Metabolites Extraction
A total of 200 grams of powder samples was extracted with methanol until submerged and left to stand for 24 hours, than filtered. The filtrate was collected and the waste was added again the same solvent until submerged. This work was repeated 3-4 times until all the estimated extractable compounds. The filtrated collected was evaporated with a rotary vacuum evaporator until all the solvent evaporates, thus obtained crude extract.
-
C. Toxicity Test
Toxicity test using bio-indicator of A. salina larvae followed the method of Meyer [12]. Media for A. salina larvae hatch was made by filtering sea water. The sea water was put into the aquarium divided into two parts, one part was made of dark covered with black paper and the other part was left open. A. salina eggs laid moderation on the part of dark and left to stand for 2 x 24 hours so that the eggs hatch into larvae that was ready for testing. Weighing 20 mg of substance to be tested was dissolved in 2 mL of n-hexane. From this solution was taken 500; 50; and 5 µL, then each inserted into a test tube and the solvent evaporated. Into each test tube added 1 mL of seawater, 50 µL of dimethyl sulfoxide, 10 larvae. Then the sea water was add to get volume of 5 mL, in order to obtain the concentration of the extract on each tube: 1000; 100; and 10 ppm. Extract with concentration of 0 ppm (without the addition of extract) was made as a control. Each test tube was covered with aluminum foil and hollowed out a bit and then left at room temperature. After 24 hours, the observation on larval mortality was carried out. The standard for assessing larvae mortality was when the larvae did not show movement for several seconds of observation [13]. The number of dead larvae were recorded, then count of LC50.
-
D. Phytochemical Test
The toxic extract were identificated the group chemical compounds including: testing of alkaloid, flavonoids, triterpenoids/steroids, polyphenols, and saponins [14].
-
III. RESULTS
-
A. Metabolites Extraction
Extraction of 200 grams of X. testudinaria sponge powder samples using methanol was produced 11.57 grams of dark green methanol extract. Acording to Sadek [15], methanol has polarity index of 5.4 and has a 100% solubility in water will dissolve the compounds that a polar to non-polar.
B.Toxicity Test
The methanol extract was assayed their toxicity by A. salina larvae. Accumulation and Percentage of deaths of larvae in the extracts were shown that Table 1.
TABLE 1. ACCUMULATION AND PERCENTAGE OF DEATHS OF LARVAE
|
Concentration |
The |
The |
Accumulation |
Percentage |
|
(ppm) |
number of |
number of |
of Deaths | |
|
live larvae |
dead larvae |
(%) |
|
1000 |
0 |
10 |
0.0 |
20.0 |
100 |
|
100 |
2 |
8 |
2.0 |
10.0 |
83 |
|
10 |
8 |
2 |
10.0 |
2.0 |
17 |
From the data in Table 1 there can be made a correlation between the log of the sample concentration and the percentage of mortality of the extract as Table 2.
TABLE 2. THE CORRELATION BETWEEN LOG OF SAMPLE CONCENTRATION AND % MORTALITY
Methanol extract
|
Log of sample concentration |
% Mortality |
|
0 |
0 |
|
1 |
19 |
|
2 |
83 |
|
3 |
100 |
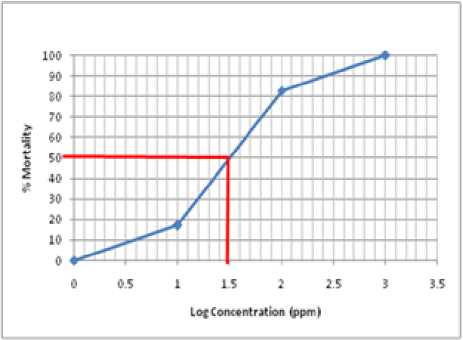
Fig. 1. The graph correlation between log of sample concentration and % mortality
From on Figure 1 above, the 50% mortality was obtained at the concentration log of 1.50. So the LC50 value for methanol extract is 31.62 ppm.
Based on the results, it showed that X. testudinaria sponge methanol extract was toxic to A. salina larvae with LC50 of 31.62 ppm. The results of this study were in line with research conducted by Zhou et al. [11]. He obtained that five compounds in the X. testudinaria sponge, namely sapinofuranone; xestospongic acid; 24-hydroperoxy-24-vinylcholesterol; Saringosterol; and 29-hydroperoxystigmasta-5,24-dien-3β-ol are toxic to A. saline larvae with LC50 values varying between 0.56 to 6.99 µM.
D. Phytochemicals Test
The toxic extract were tested phytochemically to determine the compound. Phytochemical test results was presented in Table 3.
TABLE 3. RESULTS OF PHYTOCHEMICAL TEST OF TOXIC
EXTRACT
|
Group of compounds |
Reagents |
Changes of colour |
Conclusion |
|
Alkaloids |
Dragendrof Mayer Wagner |
Red precipritate White precipitate Brown precipitate |
Positive alkaloids |
|
Flavonoids |
Na OH 10% Test Wilstatter Test Bate-Smith and Metacalf |
No changes No changes No changes |
Negative flavonoids |
|
Triterpenoids/ Steroids |
Lieberman-Burchard H2SO4 10% |
Green Green |
Positive Steroids |
|
Polyphenols |
FeCl3 10% |
Black |
Positive Polyphenol |
|
Saponins |
Uji Busa/Froth +HCl 2% |
Foam formed |
Positive Saponins |
Based on the above results it can be concluded:
-
1. Methanol extract of X. testudinaria sponge collected from Sanur beach, Bali, Indonesia was toxic against A. salina larvae with LC50 of 31.62 ppm.
-
2. The phytochemical test results showed that the toxic extract was positive containing alkaloids, steroids, polyphenols, and saponins.
Acknowledgment
We wish to express our gratitude to the Directorate of Research and Community Service, Directorate General for Research and Development, Ministry of Research, Technology and Higher Education of the Republic of Indonesia that have funded this research through Applied Product Grant Year 2017. Thank you also to the Institute for Research and Community Services, Udayana University which has been facilitated the proposal of this research so that it can be funded.
References
-
[1] E. Setyowati, U. Anggara, Sudarsono, B. Kardono, R. Rahmat, E. Meiyanto, “Cytotoxic compounds isolation of Caliapsis sponge” Indonesian Pharmacy Journal.18(4). 2007. pp. 183-189.
-
[2] A. Trianto, Ambariyanto. “Isolation of Leukemia Anticancer Compound from Sponge Agelas nakamurai and Haliclona sp” Faculty of Fisheries and Marine Sciences, Diponegoro University. 2005.
-
[3] M. J. Garson, “The Biosynthesis of Secondary Metabolits: Why is Important” In: Spons in Time and Space, Proceeding 4th International Porifera Conggress, R. W. M. Van Soest, Th. M. G. Van Kempen and J. C Braekman (eds.) Amsterdam, Netherland, 1994, pp. 428-429.
-
[4] R. J. Quinn, D. J. Tucker, “A brominated bisacetylenic acid from the marine sponge Xestospongia testudinaria” Tetrahedron Letters. 26(23). 1985. pp. 1671-1672.
-
[5] R. J. Quinn, D. J. Tucker, “Further Acetylenic Acids from the Marine Sponge Xestospongia testudinaria” J. Nat. Prod., 54 (1). 1991. Pp. 290–294.
-
[6] N. B. Pham, M. S. Butler, J. N. A. Hooper, R. W. Moni, R. J. Quinn, “Isolation of Xestosterol Esters of Brominated Acetylenic Fatty Acids from the Marine Sponge Xestospongia testudinaria” J. Nat. Prod. 62(10). 1999. pp. 1439-1442.
-
[7] W. Jiang, D. Liu, Z. Deng, N. J. de Voogd, P. Proksch, W. Lin, “Brominated polyunsaturated lipids and their stereochemistry from the Chinese marine sponge Xestospongia testudinaria” Tetrahedron, 67 (1), 2011, pp. 58-68.
-
[8] O. O. Lee, P. Y. Lai, H. W. Wu, X-J. Zhou, L. Miao, H. Wang, P. Y. Qian. “Marinobacter xestospongiae sp. nov., isolated from the marine sponge Xestospongia testudinaria collected from the Red Sea”. International Journal of Systematic and Evolutionary Microbiology. 62. 2012, pp. 1980–1985.
-
[9] L. L. Sun, C-L. Shao, J. F. Chen, Z. Y. Guo, X. M. Fu, M. Chen, Y-Y. Chen, R. Li, N. J. de Voogd, Z-G. She, Y-C. Lin, C-Y. Wan, “New bisabolane sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from the sponge Xestospongia testudinaria” Bioorganic & Medicinal Chemistry Letters. 22(3). 2012. pp. 1326-1329.
-
[10] X. C. Nguyen, A. Longeon, V. C. Pham,, F. Urvois, C. Bressy, T. T. V. Trinh, H. N. Nguyen, V. M. Phan, V. M. Chau, J. F. Briand, M. L. Bourguet-Kondracki, “Antifouling 26,27-Cyclosterols from the Vietnamese Marine Sponge Xestospongia testudinaria” J. Nat. Prod.76(7), 2013, pp. 1313–1318.
-
[11] X. Zhou, Y. Lu, X. Lin, X; Yang, B.; Yang, X; and Liu, Y. “Brominated aliphatic hydrocarbons and sterols from the sponge Xestospongia testudinaria with their bioactivities”. Chemistry and Physics of Lipids. 164: 703–706 (2011).
-
[12] B. N. Meyer, N. R. Ferrigni, Mclaughlin, “Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents “Journal of Planta Medical Research. 45, 1982, pp. 31-34.
-
[13] J. L. Carballo, Z. L. Hernandez Inda, P. Perez, M. D. Gravalos, “Comparison Between Two Brine Shrimp Assays to Detect in vitro Cytotoxicity in Marine Natural Products, BMC Biotechnology.2, 2002, pp. 1472-6570.
-
[14] J. B. Harborne, “Phytochemical methods : A Guide to Modern Techniques of Plant Analysis” London, Chapman and Hall Ltd., 1987, pp. 33-204.
-
[15] P. Sadek, The HPLC Solvents Guide. Wiley Interscience. 2002.
4
Discussion and feedback