STEROIDS FROM THE SUPER RED DRAGON FRUIT (Hylocereus costaricensis)
on
p-ISSN 1907-9850
e-ISSN 2599-2740
DOI: https://doi.org/10.24843/JCHEM.2019.v13.i02.p17
STEROIDS FROM THE SUPER RED DRAGON FRUIT (Hylocereus costaricensis)
-
H. Supriadi1, S. Salam1, F. F. Abdullah2, A. Subarnas3, R. Sidik3, U. Supratman1,4,*, Y. Shiono5
-
1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Padjadjaran, Jatinangor 45363, Indonesia.
-
2Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Garut, Garut 44151, Indonesia
-
3Faculty of Pharmacy, Universitas Padjadjaran Jatinangor 45363, Indonesia.
-
4Central Laboratory, Universitas Padjadjaran, Jatinangor 45363, Indonesia.
-
5Department of Food, Life, and Environmental Science, Faculty of Agriculture, Yamagata University, Tsuruoka, Yamagata 997-8555, Japan
*e-mail: unang.supratman@unpad.ac.id
ABSTRAK
Dua senyawa steroid, 7α-Hydoxy β-sitosterol (1) dan β-sitosterol (2), telah diisolasi dari ekstrak etil asetat Buah Naga Merah Super (Hylocereus costaricensis). Struktur kimia senyawa 1 dan 2 diidentifikasi berdasarkan data-data spektroskopi meliputi, UV, IR, NMR-1D, NMR-2D dan massa serta perbandingan data spektra dari penelitian sebelumnya. Senyawa 1 dan 2 pertama kali dilaporkan pada buah naga merah (Hylocereus costaricensis).
Kata kunci: 7α-Hydoxy β-sitosterol, β-sitosterol, Hylocereus costaricensis, steroids.
ABSTRACT
Two steroids compounds, 7α-Hydoxy β-sitosterol (1) and β-sitosterol (2), have been isolated from ethyl acetate extract of the fresh Super Red Dragon Fruit (Hylocereus costaricensis).The chemical structure of compounds 1 and 2 were identified by spectroscopic data including UV, IR, NMR-1D, NMR-2D and mass as well as by comparing with previously reported spectral data. Compounds 1 and 2 were reported for the first time from dragon fruit (Hylocereus costaricensis).
Keywords: 7α-Hydoxy β-sitosterol, β-sitosterol, Hylocereus costaricensis, steroids.
INTRODUCTION
Steroids is an important class of secondary metabolites, widely widespread in plants, animals, marines as well as fungi and have similarity to cholesterol in structure (Saeidnia et al., 2014), including β-sitosterol, campesterol, stigmasterol and cycloartenol (Ostlund, 2002). Sterols, especially β-sitosterol was reported to have interesting activity including anti-inflammatory (Prieto et al., 2006), inducing apoptosis (Chai et al., 2008; Park et al., 2007; Ju et al., 2004), chemoprotective or chemopreventive effects (Ovesna et al., 2004), hypocholesterolemic (Zak et al., 1990), angiongenic effect (Moon et al., 1999), anti-diabetic (Gupta et al., 2011;
Jamaluddin et al., 1994; Radika et al., 2013), and anti-oxidant (Baskar et al., 2012; Vivancos and Moreno, 2005).
Super Red Dragon fruit (Hylocereus costaricensis), known in Indonesia as “Naga Merah Super” is a promising tropical fruit which can be cultivated in different tropical and subtropical parts of the world such as Southeast Asia, and Central and South America. The demand for Super Red Dragon Fruit extensively increases and the fruit today can be found on almost all exotic fruit markets around the world (Salakpetch, 2000; Mohd, 2010).
Previous phytochemical studies on the species of H. costaricensis have revealed the presence mostly of polyphenolic compounds
with interesting biological activities, including (Strack et al., 2003; Karamaae et al., 2006; Wong and Siow, 2015; Wybraniec et al., 2001).
Although polyphenolic compounds of this species have been investigated previously, the steroid composition of H. costaricensis is yet to be reported. The isolation and structure identification of these isolated compounds are described herein.
MATERIAL AND METHODS
General Experimental Procedure
Melting points were measured on an electrothermal melting point apparatus and are uncorrected. The IR spectra were recorded on a Perkin-Elmer spectrum-100 FT-IR in KBr. Mass spectra were obtained with a Synapt G2 mass spectrometer instrument. NMR data were recorded on a Jeol ECZ-500 spectrometer at 500 MHz for 1H and 125 MHz for 13C, and TMS as internal standard. Column chromatography was conducted on silica gel 60 (Kanto Chemical Co., Inc., Japan). TLC plates were precoated with silica gel GF254 (Merck, 0.25 mm) and detection was achieved by spraying with 10% H2SO4 in ethanol, followed by heating.
Plant material
The fresh fruit of H. costaricensis were collected in Plantation Plant at Cicalengka District, West Java Province, Indonesia in April 2017. The plant was identified by the Mr. Joko Kusmoro, staff of the Laboratory of Plant Taxonomy, Department of Biology, Universitas Padjadjaran and a voucher specimen was deposited at the herbarium.
Extraction and isolation
The fresh fruit (25 kg) was extracted with methanol (30 L) at room temperature for 3 days. After removal of the solvent under vacuum, the concentrated of MeOH extract (120.5 g) was first suspended in H2O and then partitioned with n-hexane, EtOAc, and n-BuOH, successively. Evaporation resulted in the crude extracts of n-hexane (10.3 g), EtOAc (30.4 g), and n-BuOH (21.6 g), respectively. The n-hexane soluble fraction (10.3 g) was fractionated by vacuum liquid chromatography on silica gel 60 using a gradient n-hexane and EtOAc to give nine fractions (A–I). Fraction A (6 g) was chromatographed on a column of
silica gel, eluted successively with a gradient of n-hexane–CH2Cl2 (10:0–1:1) to give ten subfractions (A01–A10). Subfraction A03 was chromatographed on a column of silica gel, eluted with n-hexane:CHCl3 (9:1) to give 1 (12.4 mg). Fraction B (12.4 g) was fractionated by column chromatography on silica gel using a gradient n-hexane and EtOAc to give eight fractions (J–Q). Fraction K (927.6 mg) was chromatographed on a column of silica gel, eluted successively with a gradient of n-hexane–EtOAc (10:0–0:10) to give 2 (10.2 mg).
RESULT AND DISCUSSION
The phytochemical test by using Lieberman-Buchard reagents for the n-hexane extract showing the presence of steroids. By using phytochemical test to follow separations, the n-hexane fraction was separated by column chromatography over silica gel by gradient elution. The fractions were repeatedly subjected to normal-phase column chromatography on silica gel to produce two steroids 1 and 2 (Figure 1).
7α-Hydroxy-β-sitosterol (1). White needlelike crystals, m.p. 138-140 oC; IR (KBr) νmax 3450, 2940, 2860, 1469, 1365, 1045 cm-1; 1H-NMR (CDCl3, 500 MHz), δH (ppm): 1.70 (1H, m, H-1a), 1.72 (1H, m, H-1b), 1.48 (1H, m, H-2a), 1.58 (1H, m, H-2b), 3.50 (1H, m, H-3), 2.20 (1H, m, H-4a), 2.41 (1H, m, H-4b), 5.43 (1H, t, J=5.2, H-6), 4.21 (1H, m, H-7), 0.89 (1H, m, H-8), 1.45 (1H, m, H-9), 1.40 (1H, m, H-11a), 1.45 (1H, m, H-11b), 1.18 (1H, m, H-12a), 1.30 (1H, m, H-12b), 0.90 (1H, m, H-14), 1.54 (1H, m, H-15a), 1.06 (1H, m, H-15b), 1.45 (1H, m, H-16a), 1.25 (1H, m, H-16b), 1.28 (1H, m, H-17), 1.15 (3H, s, Me-18), 0.71 (3H, s, Me-19), 1.84 (1H, m, H-20), 0.90 (3H, d, J=6.2, Me-21), 2.14 (1H, m, H-22a), 2.23(1H, m, H-22b), 2.17 (1H, m, H-23a), 2.33 (1H, m, H-23b), 1.60 (1H, m, H-24), 1.82 (1H, m, H-25), 0.86 (3H, d, J=6.5 Hz, Me-26), 0.85 (3H, d, J=5.2 Hz, Me-27), 1.30 (1H, m, H-28a), 1.48 (1H, m, H-28b), 1.81 (3H, t, J=5.2 Hz, Me-29); 13C-NMR (CDCl3, 125 MHz), see Table 1; TOFMS (negative ion mode) m/z 413.3811 [M-H]-, (calcd. C29H49O-, m/z 413.3844).
β-sitosterol (1). White needle-like crystals, m.p. 143-145 oC; IR (KBr) νmax 3440, 2935,
2869, 1452, 1368, 1045 cm-1; 1H-NMR (CDCl3, 500 MHz), δH (ppm): 1.75 (1H, m, H-1a), 1.82 (1H, m, H-1b), 1.65 (1H, m, H-2a), 1.72 (1H, m, H-2b), 3.65 (1H, m, H-3), 2.34 (1H, m, H-4a), 2.38 (1H, m, H-4b), 5.21 (1H, t, J=5.0, H-6), 2.56 (1H, m, H-7a), 2.72 (1H, m, H-7b), 0.90 (1H, m, H-8), 1.48 (1H, m, H-9), 1.50 (1H, m, H-11a), 1.55 (1H, m, H-11b), 1.25 (1H, m, H-12a), 1.35 (1H, m, H-12b), 0.96 (1H, m, H-14), 1.64 (1H, m, H-15a), 1.16 (1H, m, H-15b), 1.60 (1H, m, H-16a), 1.41 (1H, m, H-16b), 1.32 (1H, m, H-17), 1.35 (3H, s, Me-18), 0.81 (3H, s, Me-19), 1.84 (1H, m, H-20), 0.96 (3H, d, J=6.2, Me-21), 2.35 (1H, m, H-22a), 2.50 (1H, m, H-22b), 2.26 (1H, m, H-23a), 2.53 (1H, m, H-23b), 1.90 (1H, m, H-24), 1.92 (1H, m, H-25), 0.96 (3H, d, J=6.5 Hz, Me-26), 0.88 (3H, d, J=5.2 Hz, Me-27), 1.46 (1H, m, H-28a), 1.57 (1H, m, H-28b), 1.91 (3H, t, J=5.2 Hz, Me-29); 13C-NMR (CDCl3, 125 MHz), see Table 1; TOFMS (negative ion mode) m/z 413.3862 [M-H]-, (calcd. C29H49O-, m/z 413.3789).
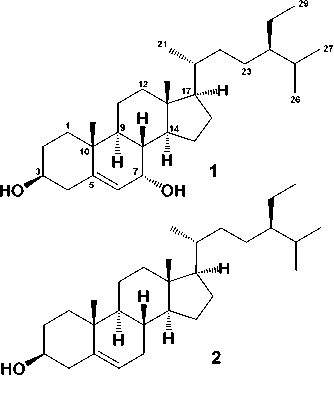
Figure 1. Chemical Structure of 1 and 2
Compound 1 was obtained as a white needle-like crystal. The TOFMS spectrum showed [M-H]+ m/z 413.3811 (calcd. m/z 413.3844), which coresponded to the molecular formula C29H50O and thus required requiring hydrogen deficiency index of five, originating from one pairs of C sp2 and the remaining tetracyclic stigmastane-type steroid. The IR spectra showed absorption peaks at 3450 cm-1 (OH), 2940 and 2860 cm-1 (aliphatic), 1469 cm-1 (C=C), 1365 and 1240 cm-1 (gem-dimethyl groups), and 1045 cm-1 (CO). The 1H-NMR (CDCl3 500 MHz) spectrum
showed the presence of six methyl groups, two tertiary methyl groups resonating at δH 1.15 (Me-18), 0.71 (Me-19), three secondary methyl groups resonating at δH 0.90 (3H, d, J = 6.2 Hz, Me-21), 0.86 (3H, d, J = 6.5 Hz, Me-26), and 0.85 (d, J = 5.2 Hz, Me-27), one primary methyl group resonating at δH 1.81 (t, J = 5.2 Hz, Me-29), which was indicate the presence of stigmastane-type steroid skeleton (Cayme and Ragasa, 2004, Farabi et al., 2017). One olefinic methine group, resonating at δH 5.43 (1H, t, J=5.2, H-6), and oxymethine group resonating at δH 3.50 (1H, m, H-3) also observed at 1H NMR spectra. The proton pairing was also confirmed with the 1H-1H COSY spectrum (Figure 2). 1H-1H COSY cross peak observed at H-2/H-3/H-4 indicated that position of hydroxy group at C-3. The cross peak also observed at H-6/H-7/H-8 that indicated the position of double bond at C-5/C-6. The 13C-NMR (CDCl3 125 MHz) and HMQC and DEPT 135° spectra showed the presence of six methyl groups, one olefinic methine, one olefinic quartenary carbon, and a oxygenated methine group, resonating at δC 71.5 (C-3) and 74.5 (C-7), indicated the characteristic of stigmastane-type steroid (Cayme and Ragasa, 2004; Farabi et al., 2017). These functionalities accounted for one of total five degree of unsaturations. The remaining four degrees of unsaturation were consistent with the stigmastane-type steroid. Correlation of H-2 and H-4 to an oxygenated carbon at δC 71.5, indicated that hydroxyl group attached at C-3. Another hydroxyl group was located at C-7 based on the correlation of H-6 and H-8 to oxygenated carbon at C-7 (δC 74.5). A comparison of the NMR data of 1 with the data for 7α-Hydroxy-β-sitosterol (Chaturvedula and Prakash, 2012; Farabi et al., 2017), revealed that the structure of the two compounds were very similar, consequently compound 1 was identified as a 7α-Hydroxy-β-sitosterol, which shown in this plant for the first time.
Compound 2 was obtained as a white needlelike crystal. The IR spectra showed absorption peaks at 3480 cm-1 (OH), 2960 and 2865 cm-1 (aliphatic), 1472 cm-1 (C=C), 1382 and 1238 cm-1 (gem-dimethyl groups), and 1040 cm-1 (C-O). The NMR spectra of 2 very similar with 1, the main difference was that compound 2 the absence of hydroxyl group at [δH 4.21 (1H, m, H-7), δC 74.5] and the presence of the methylene signal at [δH 2.56 (1H, m, H-7a),
2.72 (1H, m, H-7b), δC 32.4], suggested that 2 is 7-dehydroxy derivative of 1. In comparison of 2 with literature data of a stigmast-5-en-3β-ol (β-sitosterol) (Chaturvedula and Prakash, 2012; Harneti et al., 2014; Farabi et al., 2017), showed good agreement, therefore compound 2 was identified as a stigmast-5-en-3β-ol (β-sitosterol), which shown in this plant for the first time.
Table 1. NMR data for compounds 1 and 2*
|
Position of Carbon |
1 δC (mult.) |
2 δC (mult.) |
|
1 |
36.6 (t) |
37.1 (t) |
|
2 |
31.4 (t) |
32.6 (t) |
|
3 |
71.5 (d) |
72.2 (d) |
|
4 |
42.8 (t) |
42.3(t) |
|
5 |
145.2 (s) |
146.1 (s) |
|
6 |
122.5 (d) |
123.6 (d) |
|
7 |
74.5 (d) |
34.6 (t) |
|
8 |
32.4 (d) |
31.4 (d) |
|
9 |
50.6 (d) |
51.6 (d) |
|
10 |
36.6 (s) |
37.6 (s) |
|
11 |
21.5 (t) |
22.5 (t) |
|
12 |
40.2 (t) |
41.2 (t) |
|
13 |
42.6 (s) |
41.6 (s) |
|
14 |
57.4 (d) |
58.4 (d) |
|
15 |
26.7 (t) |
27.7 (t) |
|
16 |
28.8 (t) |
27.9 (t) |
|
17 |
56.4 (d) |
56.9 (d) |
|
18 |
12.6 (q) |
12.2 (q) |
|
19 |
19.1 (q) |
18.5 (q) |
|
20 |
36.2 (d) |
35.2 (d) |
|
21 |
19.0 (q) |
18.5 (q) |
|
22 |
34.4 (t) |
34.2 (t) |
|
23 |
26.0 (t) |
26.5 (t) |
|
24 |
45.4 (d) |
44.8 (d) |
|
25 |
29.6 (d) |
29.4 (d) |
|
26 |
19.0 (q) |
18.5 (q) |
|
27 |
20.4 (q) |
20.2 (q) |
|
28 |
23.0 (t) |
22.5 (t) |
|
29 |
12.5 (q) |
12.4 (q) |
*measured in CDCl3 (500 MHZ for 1H and 125 MHz for 13C)
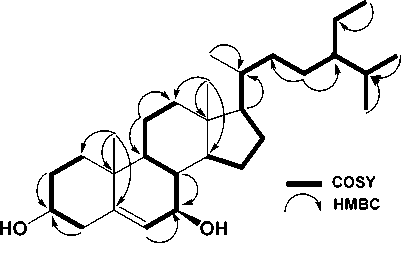
Figure 2. Selected 1H-1H COSY and HMBC correlation for compound 1
CONCLUSIONS
Two steroids have been isolated from the fresh fruit of H. costaricensis and identified by spectroscopic methods as 7α-Hydroxy-β-sitosterol and β-sitosterol (2), The investigation of these steroids were shown in this species for the first time.
ACKNOWLEDGMENTS
We thank Dr. Ahmad Darmawan, M.Si and Dr. Sofa Fajriah, M.Si in the Research Center for Chemistry, Indonesian Science Institute, for NMR measurements.
REFERENCES
Baskar, A. A., K. S. Al Numair, M. G. Paulraj, M. A. Alsaif, M. Al Muamar, and S. Ignacimuthu. 2012. β-Sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. Journal of Medicinal Food. 15: 335-343.
Cayme, J., Ragasa, C. 2004. Structure elucidation of β-stigmasterol and β-sitosterol from Sesbania grandiflaora (Linn). Pers. and β-carotene from Heliotropium indicum Linn by NMR spectroscopy. J. Kimika. 20: 5-12.
Chai, J. W., U. R. Kuppusamy, and M. S. Kanthimathi. 2008. Beta-sitosterol induces apoptosis in MCF-7 cells. Malaysian Journal of Biochemistry Molecular Biology. 16: 28-30.
Chaturvedula, V.S.P and Prakash, I. 2012. Isolation of stigmasterol and □ -sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 1: 239-242.
Farabi, K., Harneti, D., Nurlelasari., Maharani, R., Hidayat, A.C., Supratman, U., Awang, K., Shiono, Y. 2017. Cytotoxic Steroids from the Bark of Aglaia argentea (Meliaceae). CMU J. Nat. Sci. 16(4): 293-306.
Gupta A., A. K. Sharma, M. P. Dobhal, M. C. Sharma, and R. S. Gupta. 2011. Antidiabetic and antioxidant potential of β-sitosterol in treptozotocin-induced experimental hyperglycemia. Journal of Diabetes. 3: 29-37.
Harneti, D., Supriadin, A., Ulfah, M., Safari, A., Supratman, U., Awang, K., Hayashi, H., 2014. Cytotoxic constituents from the bark of Aglaia eximia (Meliaceae). Phytochem. Lett. 8: 28–31.
Jamaluddin F., S. Mohamed, M. N. Lajis. 1994. Hypoglycaemic effect of Parma speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chemistry. 49: 339-345.
Jamilah, B., C.E. Shu, M. Kharidah, M.A. Dzulkifly, and A. Noranizan, 2011. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. International Food Research Journal. 18: 279-286.
Ju, Y. H., L. M. Clausen, K. F. Allred, A. L. Almada, and W. G. Helferich. 2004. β-sitosterol, β-sitosterol glucoside, and a mixture of β-sitosterol and β-sitosterol glucoside modulate the growth of estrogen-responsive breast cancer cells In vitro and in ovariectomized athymic mice. Journal of Nutrition. 134: 11451151.
Karamaae, K., Kosinska, A., Pegg, R.B. 2006. Content of gallic acid in selected plant extract. Pol. J. Food. Nutr. Sci., 15(56): 55-58.
Mohd, M.H., 2010. Diversity of Fusarium semitectum (berkeley and ravenel) associated with red-fleshed dragon fruit (Hylocereus polyrhizus [weber] britton and rose) in Malaysia, Universiti Sains Malaysia.
Moon E. J., Y. M. Lee, O. H. Lee, M. J. Lee, S. K. Lee, and M. H. Chung. 1999. A novel angiogenic factor derived from Aloe vera gel: beta-sitosterol, a plant sterol. Angiogenesis. 3: 117-123.
Ostlund, R. E., J. B. Mcgill, C. Zeng, D. F. Covey, J. Stearns, and W. F. Stenson. 2002. Gastrointestinal absorption and plasma kinetics of soy 5-phytosterols and phytostanols in humans. American Journal of Physiology and Endocrinol Metabolism. 282: 911–916.
Ovesna Z., A. Vachalkova, and K. Horvathova. 2004. Taraxasterol and beta-sitosterol: new naturally compounds with chemoprotective/chemopreventive effects. Neoplasma. 51: 407-414.
Park, C., D. O. Moon, C. H. Rhu, B. T. Choi, W. H. Lee, G. Y. Kim, and Y. H. Choi. 2007. β-sitosterol induces
antiproliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biological & Pharmaceutical Bulletin. 30: 1317-1323.
Prieto, J. M., M. C. Recio, and R. M. Giner. 2006. Anti-inflammatory activity of β-sitosterol in a model of oxazolone induced contact-delayed-type
hypersensitivity. Boletín
Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 5: 57-62.
Radika, M. K., P. Viswanathan, and C. V. Anuradha. 2013. Nitric oxide mediates the insulin sensitizing effects of β-sitosterol in high fat diet-fed rats. Nitric Oxide. 32: 43-53.
Saeidnia, S., A. Manayi, A. R. Gohari, and M. Abdollahi. 2014. The Story of Betasitosterol - A Review. European Journal of Medicinal Plants. 4(5): 590-609.
Salakpetch, S. 2000. Tropical Fruit Production of Thailand, pp. 1-12, In M. A. Nagao, (ed.) Hawaii Tropical Fruit Growers Tenth Annual International Tropical Fruit Conference, Hilo, Hawaiian Hotel, Hilo, Hawai
Strack, D., Vogt, T., Schliemann et al. 2003. Recent advances in betalain research. Phytochemistry. 62(3):247-269.
Wong, Y.M and Siow, L.F. 2015. Effects of heat, pH, antioxidant, agitation and light on betacyanin stability using red-fleshed dragon fruit (Hylocereus polyrhizus) juice and concentrate as models. J. food Sci. Technol. 52(5): 3086-3092.
Wybraniec, S., Platzner, I., Geresh, S., Gottlieb, H.E., Haimberg. 2001. Betacyanins from vine cactus Hylocereus polyrhizus. Phytochemitsry. 58(8): 1209-1212.
Vivancos, M., and J. J. Moreno. 2005. β-Sitosterol modulates antioxidant enzyme response in RAW 264.7 macrophages. Free Radical Biology and Medicine. 39: 91-97.
Zak A., M. Zeman, D. Vitkova, P. Hrabak, and E. Tvrzicka. 1990. Beta-sitosterol in the treatment of hypercholesterolemia. Casopis éLkaru Ceských. 129: 13201323.
233
Discussion and feedback