MOLECULAR MODELING OF CATIONIC PORPHYRINS AS LIGAD OF RADIOPHARMACEUTICAL KIT
on
JURNAL KIMIA 5 (1), JANUARI 2011 : 94-100
MOLECULAR MODELING OF CATIONIC PORPHYRINS AS LIGAD OF RADIOPHARMACEUTICAL KIT
Ni Made Pitri Susanti1,2), Rahmana E. Kartasasmita2), Amir Musadad2), and Daryono H. Tjahjono2)
1)Department of Pharmacy, Faculty of Matheatics and Natural Science, Udayana University, Bukit Jimbaran, Badung 80363, Indonesia (E-mail: p_susanti@yahoo.com)
2)School of Pharmacy, Bandung Institute of Technology, Jalan Ganesha 10 Bandung 40132, Indonesia
ABSTRAK
Porfirin kationik dan interaksinya dengan DNA telah menjadi perhatian penting dalam pengembangan fotosensitizer dalam teknik terapi fotodinamik pada pengobatan kanker. Namun demikian, teknik terapi ini memiliki kekurangan dalam mendokumentasikan secara fotografik fluoresensi yang teramati secara endoskopi[. Penelitian ini bertujuan mengamati perubahan molekular senyawa porfirin kationik yang dilabel dengan radionuklida pemancar radiasi gama (γ) dan partikel beta (β) beserta afinitasnya terhadap DNA secara teoritik. Model molekul 5,10,15,20-tetrakis-[3,4-bis(karboksimetilenoksi) imidazolium] porfirin (T3,4BCImP), 5,10,15,20-tetrakis-[3,4-bis (karboksimetilenoksi) pirazolium] porfirin (T3,4BCPzP) serta kompleks hasil pelabelan dengan radionuklida Tc dan Re pada gugus karboksinya diopti masi dengan metode teori fungsi densitas (density functional theory/DFT), sedangkan muatan atom dihitung menggunakan natural population analysis (NPA). Energi orbital molekul juga dihitung menggunakan metode dan parameter yang sama. Hasil penelitian menunjukkan bahwa molekul Tc-T3,4BCPzP memiliki fotosensitifitas dan afinitas yang terbaik terhadap DNA dibandingkan molekul lainnya. Gugus karboksilat pada substituen meso memungkinkan T3,4BCPzP dan T3,4BCImP dapat di-label dengan radionuklida Tc dan Re sebagai kandidat kit radiofarmasi.
Kata kunci : porfirin kationik, radiolabel, DFT, energi orbital molekul
ABSTRACT
Cationic porphyrins and their interactions with DNA have become an important concern due to its role as a photosensitizer in photodynamic therapy for cancer treatment. However, this therapy technique has the disadvantage, i.e. its inability to document photographically the fluorescence observed endoscopically. The present research aims to observe the change in molecular level of cationic porphyrins which labeled by radionuclides emitting β particle and γ radiation. Molecular models of 5,10,15,20-tetrakis-[3.4-bis (carboxymetylenoxy) imidazoliumyl] porphyrin (T3,4BCImP), 5,10,15,20-tetrakis-[3,4-bis (carboxymetylenoxy) pirazoliumyl] porphyrin (T3,4BCPzP) and its complexes which labeled by Tc and Re radionuclides were optimized and calulated by density functional theory methods (DFT). Atomic charges were calculated with natural population analysis/NPA method. The calculation result showed that Tc-T3,4BCPzP has the highest photosensitivity and the strongest affinity to DNA. Carboxylate groups of meso-subtituent of porhyrins lead to label cationic porphyrins by Tc and Re as radiopharmaceutical ligand candidates .
Keywords : cationic porphyrins, radiolabelled, DFT, molecular orbital energy
INTRODUCTION
Photodynamic therapy (PDT) is a novel technique in cancer therapy that does not affecting normal cells. The procedure requires explosure of cells or tissues to a photosensitizer followed by irradiation with visible light of appropriate wave length, usually in the red or near infra-red region and compalible with the absorption spectrum of the photosensitizer (Tjahjono, 2006 ; Oleinick et al, 2002). Porphyrin and its derivatives are photosensitizer that currently being developed. Porphyrin-based compounds have good absorption in visible and infra-red region, relatively high fluorescence and high affinity to DNA. Selectively, porphyrin can accumulate on the surface of tumor cells, become internalized, bind to DNA and then induce DNA strand cleavage (Bennet et al, 200).
Cationic porphyrins and their interactions with DNA are of interest from the view point of their role in biological systems. It has been reported that they act as an human telomerases inhibitor, a receptor for peptides and a DNA cleaver (Tjahjono, 2006). Although PDT is clinically well exploited, this therapy technique still has the disanvantage, i.e. its inability to document photographically the fluorescence as observed endoscopicaly. This disadvantage could be posibbly circumvented if porphyrin and their derivatives could be radiolabelled with suitable radionuclides and therefore, development of radiolabelled porphyrin has recieved considerable attention (Das et al, 2008). However, radiolabeling can result in molecular change of porphyrin.
Technetium, in form of metastable isotop 99mTc, is the most widely used radionuclide in radiolabeled compound for diagnostic and therapy purposes. The extensive uses of 99mTc emerges from its favourable nuclear properties (t½ = 6.02 h, Eγ = 140 keV) which is suitable for detection with high efficiency resulting in low radiation exposure to patiens (Shetty et al, 1996; Boros et al, 2009). As technetium (Tc) and rhenium (Re) are chemical congeners, Re can exhibits the same chelation and radiolabeling chemistry as Tc. 188Re has high-energy β- emission (Emax = 2.12
MeV), γ emission (Eγ = 155keV) and
appropriate half-life of 16.9 h (Jia et al, 2008).
The present research aims to observe the molecular change of cationic porphyrins which are labeled by radionuclides emitting β particles or γ radiation and study its possibility as ligand for radiopharmaceitucal kit.
MATERIALS AND METHOD
Materials
Compiuter equipped with Gauss View 03 program.
Experimental
Molecular models of 5,10,15,20-tetrakis[3,4-Bis(carboxymethylenoxy)imidazo-liumyl] porphyrin (T3,4BCImP), 5,10,15,20-
tertakis[3,4-bis(carboxymethylenoxy)pirazoliu-myl] porphyrin (T3,4BCPzP) and their
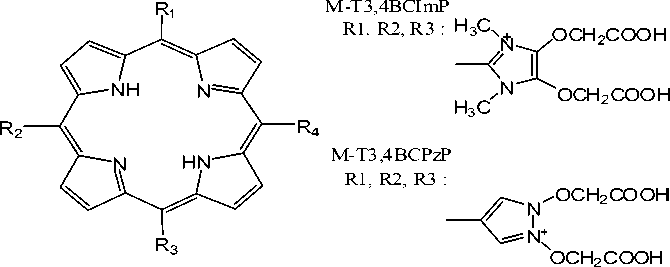
complexes which are labeled by technetium (Tc) and rhenium (Re) radionuclides were constructed using Gauss View 03 program. The structural sketch of all studied porphyrins with no symetry were shown in Figure1.
The full geometry optimization were computed by density functional theory (DFT)-B3LYP method with 6-31g* basis set level for T3,4BCImP and T3,4BCPzP, and Lanl2dz basis set level for its complexes which are labeled by Tc and Re. A single point calculation was also further performed based on the basis set level. Orbital plotting were performed to observe electron distribution in molecules. All calculation were performed with Gaussian 03 (B-04) program-package (Frisch et al, 2003).
RESULTS AND DISCUSSION
Molecular models of cationic porphyrins were optimized to obtain molecular geometry with the smallest total energy. The smaller the total energy of a molecule, the more stable the molecule. The calculation result shows that the T3,4BCImP molecule has a smaller total energy than T3,4BCPzP molecule, whereas Tc and Re complexes of each molecule have the same total energy.
Each molecular orbital (MO) has a energy level, HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) energies, which is important to determine the reactivity of molecule. HOMO-LUMO energy gap can be
used to measure the ease of molecule to excite. The smaller the gap of energy, the easier the excitation of molecules. HOMO and LUMO energy of each molecule obtained from the single point energy (SPE) calculation on the optimized molecules shows in table 1.
R4 :
T3,4BCImP
R1, R2, R3, R4 :
T3,4BCPzP
R1,R2,R3,R4 :
R4 :
H3C OCH2COOH

OCH2COOH +
N OCH2COOH
H3C

OCH2COOH
OM Z O
H2 O
H2 ZO
O
O
M : Tc, Re
Fig. 1. Structure of cationic porphyrins
Table 1. Homo and LUMO energy
|
Energy (eV) |
Molecule | |||||
|
T3,4BCImP |
3,4BCPzP Tc-T3,4BCImP Re-T3,4BCImP |
Tc-T3,4BCPzP |
Re-T3,4BCPzP | |||
|
HOMO-4 |
-13,93 |
-14,77 |
-16.10 |
-16,03 |
-16,43 |
-16,37 |
|
HOMO-3 |
-13,91 |
-14,10 |
-16.03 |
-15,91 |
-16,20 |
-16,14 |
|
HOMO-2 |
-13,88 |
-13,98 |
-15.79 |
-15,77 |
-16,17 |
-16,08 |
|
HOMO-1 |
-13,85 |
-13,06 |
-15.62 |
-15,57 |
-15,49 |
-15,42 |
|
HOMO |
-13,84 |
-12,96 |
-15.56 |
-15,52 |
-15,39 |
-15,33 |
|
LUMO |
-11,13 |
-10,19 |
-14.07 |
-13,53 |
-14,66 |
-14,28 |
|
LUMO+1 |
-11,02 |
-10,13 |
-13.85 |
-13,36 |
-14,38 |
-14,02 |
|
LUMO+2 |
-9,44 |
-9,45 |
-13.44 |
-13,25 |
-13,97 |
-13,89 |
|
LUMO+3 |
-8,51 |
-9,44 |
-13.24 |
-13,21 |
-13,69 |
-13,59 |
|
LUMO+4 |
-8,46 |
-9,39 |
-12.98 |
-12,81 |
-13,35 |
-13,21 |
|
HOMO- | ||||||
|
LUMO |
2,71 |
2,78 |
1.48 |
1,99 |
0,73 |
1,05 |
According to frontier molecular orbital theory, the higher occupied MOs energy of the one donor molecule and the lower unoccupied MOs energy of the acceptor are advantageous to the interaction between the two molecules, because are electrons more easily transferred from the occupied MOs of donor to the unoccupied MOs of acceptor (Fleming, 1976).
Based on calculation of DNA base-pairs with backbone that had been carried out by Kurita and Kobayashi (Kurita and Kobayashi, 2000), it is known that the energies of HOMO and occupied MOs near the HOMO (HOMO–n; n = 1 - 4) of DNA are – 1.27, – 1.33, – 1.69, – 1.79 and – 1.98 eV respectively, while its LUMO energy is 1.14 eV. The data in table 1 shows that the HOMO and HOMO–n (n = 1 - 4) energies of all porphyrin molecules are much lower than those HOMO and HOMO–n (n = 1 -4) energies of the DNA base-pairs, so that, in interaction, DNA will act as electron donor. In addition, LUMO and unoccupied MOs near the LUMO (LUMO+n; n = 1 - 4) energies of all molecules are also much lower than the HOMO and HOMO–n (n = 1 - 4) energies of DNA. Therefore, porphyrins can interacts with DNA strongly and should be good electron acceptor when binding to DNA. Moreover, the calculation
result shows that the LUMO and LUMO+n (n = 1 - 4) energies of Tc and Re T3,4BCImP and also Tc and Re T3,4BCPzP are lower than their parent molecule. HOMO-LUMO energy gap of radiolabeled molecules are also smaller than their parent molecule. Thus, it can be conclude that addition of radionuclide on carboxylate group of meso substituent of cationic porphyrins will improve their photosensitivity and affinity to DNA. So that, these molecules may suitable to be developed as radiopharmaceutical ligand candidates. Of all the tested molecules, Tc-T3,4BCPzP has the lowest LUMO energy and the smallest HOMO-LUMO energy gap, so it can be stated that this molecule has the highest fotosensitivity and affinity to DNA.
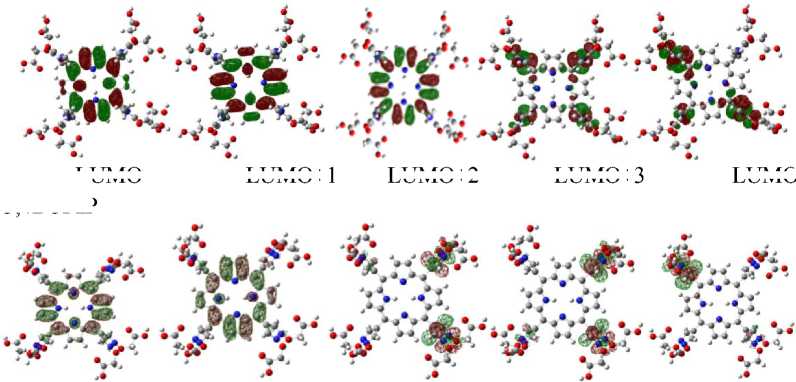
On the other hand, Kurita and Kobayashi (Kurita and Kobayashi, 2000) also states that the HOMO and HOMO–1 orbitals of DNA mostly populated on base-pairs and HOMO–n (n = 2 -4) mostly on phosphate groups. This explain the interaction mode that may occur between porphyrins and DNA. Orbital plotting result of each tested molecules are showed in figure 2 and figure 3.
T3,4BCImP
LUMO+1
LUMO+2
LUMO+3
LUMO T3,4BCPzP
LUMO
LUMO+1
LUMO+2
LUMO+3
LUMO+4
LUMO+4
Fig. 2. Some frontier orbital contour of cationic porphyrins calculated at the level B3LYP/6-31g*
Tc-T3,4BCImP

LUMO+1
LUMO+2
LUMO+3
LUMO+4
LUMO Re-T3,4BCImP
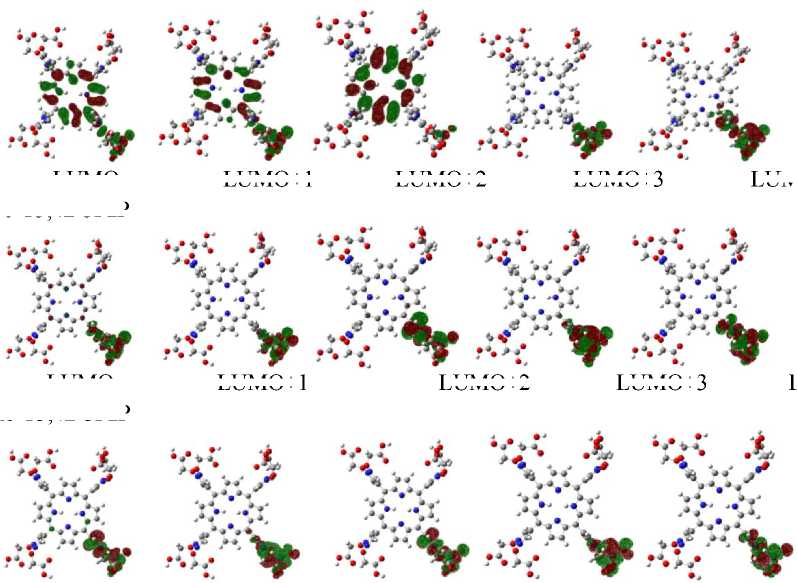
LUMO+1
LUMO+2
LUMO+3
LUMO+1
LUMO+2
LUMO+3
LUMO+4
LUMO
LUMO+1
LUMO+2
LUMO+3
Fig. 3. Some frontier orbital contour of radiolabeled cationic porphyrins calculated at the level B3LYP/lanl2dz
LUMO Tc-T3,4BCPzP
LUMO Re-T3,4BCPzP
LUMO+4
LUMO+4
Figure 2 and 3 shows that LUMO and LUMO+n (n = 1 - 2) of T3,4BCImP have much component populated on porphyrin core, while LUMO+n (n = 3 - 4) are mainly from the imidazole substituent. LUMO and LUMO+1 of T3,4BCPzP are mostly populated on porphyrin core, while LUMO+n (n = 2 - 4) are mainly from the pirazole substituent. For cationic porphyrins with radionuclides, LUMO and LUMO+n (n = 1 - 4) are mostly populated on the meso substituent. These indicate that the interaction of cationic porphyrins and DNA mainly through π-
π stacking between porphyrin plane and DNA base-pairs if the molecules can intercalated into DNA and stabilized by electrostatic interaction between positively charged of meso substituent of cationic porphyrins and the negatively charged of phosphate groups in DNA backbone. The interaction of radiolabeled cationic porphyrins with DNA occurs mainly through an electrostatic interaction between positively charged of meso substituent of cationic porphyrins and the negatively charged of phosphate groups in DNA backbone.
CONCLUSION
The computation result showed that there were changes at molecular level of labeled cationic porphyrins compared to the parent molecules based on the bond distance and angde, total molecular energy and electronic distribution. HOMO and LUMO energy levels indicate that Tc-T3,4BCPzP has the highest photosensitivity and affinity to DNA. Carboxylate groups of meso substituent of T3,4BCImP and T3,4BCPzP could be labeled by Tc and Re and hence the two molecules may be suitable to develope as radiopharmaceutical ligand candidates.
ACKNOWLEDGEMENT
This research was supported by Directorate of Higher Education, Ministry of National Education, the Republic of Indonesia
REFERENCES
Boros,E., Häfeli, U. O., Patrick, B. O., Adam, M.
J. and Orvig, C., 2009, Design,
Synthesis, and Imaging of Small Amphiphilic Rhenium and
99mTechnetium Tricarbonyl Complexes, Bioconjugate Chem, 20, 1002-1009 Bennet, M., Krah, A., Wien, F., Garman, E.,
Mckenna, R., Sanderson, M., and Neidle, S., 2000, A DNA-Porphyrin Minor
Groove Complex at Atomic Resolution : The Structural Consequences of Porphyrin Ruffling, PNAS, 97(17), 94769481.
Das, T., Chakraborty, S., Sarma, H. D. and Benerjee, S., 2008, A Novel [109Pd]
Palladium Labeled Porphyrin for Possible Use in Targeted Radiotherapy, Radiochem Acta, 96, 427-433.
Fleming, I., 1976, Frontier Orbitals and Organic Chemical Reactions, John Wiley & Sons, New York, 24-33.
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JAJr., Vreven T, Kudin
KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C and Pople JA., 2003, Gaussian 03,Revision B.04, Gaussian, Inc., Pittsburgh PA.
Jia, Z., Deng, H., Pu, M. and Luo, S., 2008, Rhenium-labelled meso-tetrakis[3,4-bis(carboxymethyleneoxy)phenyl] porphyrin for Targeted Radiotherapy : Preliminary Biological Evaluation in Mice, Eur. J. Nucl. Med. Mol. Imaging, 35, 734-742.
Kurita, N. and Kobayashi, K., 2000, Density Functional MO Calculation for Stacked DNA Base-pair with Backbone, Computer and Chemistry, 24 : 351-357
Oleinick NL, Morris RL and Belichenko I., 2002, The Role of Apoptosis in Response to Photodynamic Therapy, What, Where, Why and How, Photochem. Photobiol. Sci., 1 :1-21
Shetty, S.J., Murugesan, S., Chatterjee, S.R., Banerjee, S., Srivastava, T.S., Noronha, O.P.D. and Samuel, A.M.,1996, A New 99mTc Labeled Porphyrin for Specific Imaging of Sarcoma 120 : Synthesis and Biological Study in Swiss Mouse Model, J. of Labeled Compounds and Radiopharmaceuticals, 38 (5) : 411-418
Tjahjono, D.H., 2006, Porpyrin Structure-based Molecules for Photodynamic Therapy of
Cancer, Acta Pharmaceutica Indonesia, 31 (1) : 1-12
100
Discussion and feedback