ISOLATION AND IDENTIFICATION MOMORDICIN I FROM LEAVES EXTRACT OF Momordica charantia L.
on
ISSN 1907-9850
ISOLATION AND IDENTIFICATION MOMORDICIN I FROM LEAVES EXTRACT OF Momordica charantia L.
N. M. Puspawati
Department of Chemistry Faculty of Mathematics and Natural Sciences University of Udayana, Bukit Jimbaran
ABSTRAK
Senyawa momordicin I telah berhasil diisolasi dari ekstrak diklorometana daun pare, Momordica charantia L. Isolasi dilakukan dengan memfraksionasi ekstrak diklorometana yang diikuti dengan bioassay menggunakan larva udang artemia salina, L untuk mendapatkan fraksi paling aktif. Fraksionasi dilakukan dengan kolom kromatografi phase terbalik (C18Sep-Pak) menggunakan satu seri eluen MeOH:H2O (0-100% MeOH) secara gradien. Fraksi paling aktif, F9 (80% MeOH : 20% H2O) selanjutnya dimurnikan dengan rekristalisasi menggunakan kloroform sehingga dihasilkan kristal berwarna putih dengan titik leleh 125-128oC. Dari hasil interpretasi data spectra IR, 1HNMR,13CNMR, DEPT, HCOSY, HMCQC, HMBC and MS, struktur isolat aktif dielusidasi sebagai 3,7,23-trihydroxycucurbitan-5,24-diena-19-al yang lebih dikenal dengan senyawa momordicin I.
Kata Kunci : isolasi dan identifikasii, momordicin I, Momordica charantia L., Artemia salina L.
ABSTRACT
Momordicin I has been isolated from dichloromethane extract of leaves Momordica charantia, L. The isolation was done by fractionation the dichloromethane extract followed by bioassay using artemia salina,L larvae. The crude extract was fractionated through reverse phase column chromatography (C18 Sep-Pak) with eluotropic series solution of MeOH: H2O (0-100% MeOH). The most active fraction, F9 (80% MeOH-20% H2O) was purified by recrystallisation from chloroform to yield a white crystal melted at 125-128oC. The structure of the active compound was determined by interpretation of the spectral data (IR, 1HNMR, 13CNMR, DEPT, HCOSY, HMCOSY, HMBC and MS) and its structure was elucidated as 3,7,23-trihydroxycucurbitan-5,24-dien-19-al which is known as momordicin I.
Keywords : isolation and identification, momordicin I, Momordica charantia L., Artemia salina L.
INTRODUCTION
Momordica charantia, L is found in the tropics and it is widely cultivated as vegetable crop. The fruits, leaves and roots of M. charantia have been used in Ayurweda for a number of diseases, as a bitter stomachic, laxative and anthelmentic (Begum, 1997). In India, the whole extract of the fruit is advocated in diseases of spleen, liver, rheumatism, gout, etc
(Begum, 1997). In the Philiphines, it was reported that juice extracts from the green fruit of this plants are given for chronic colitis and bacillary dysentery; whereas the juice of the leaves are given in the amount of a teaspoon for children coughs. The seeds of this plant were also reported to have potential activity as antidiabetes (Ali, 1993), antitumor (NG, 1994), and tripsin and elastase inhibitors (Hamato,1995)
Preliminary screening for cytotoxicity activity of some Balinese plant extracts showed that dichloromethane extract of leaves Momordica charantia, L was toxic against artemia salina larvae (Puspawati, 1997). Therefore, this research was done to isolate and identify the active constituent of the leaves Momordica charantia, L.
MATERIALS AND METHOD
Materials
Momordica charantia, L was collected from Gubug, Tabanan-Bali. The leaves parts were air dried, cut up and ground to become powder. Atemia salina, L (A.s) egg was provided by technical training centre UNUD. All solvents used in this research were proanalysis grade.
Method
Extraction was carried out using cold solvent. Evaporation of the solvent under reduce pressure was done on Buchi rotary evaporator. Fractionation was performed on Waters Sep-Pak classic short body catridge with C18 as stationary phase.
Melting point was determined on Gallen Kamp Capillary melting apparatus and uncorrected.
NMR spectra were recorded on Varian Gemini operating at 400 MHz. IR spectra were taken on Perkin Elmer model 1760 and mass spectra were run on VG Flat Form 2 micromass.
Bioassay using artemia salina, L (A.s) larvae was conducted according to a literature procedure (Meyer, 1982).
Experimental
Isolation and identification
The powder sample was extracted with dichloromethane. The filtrate was evaporated to give crude dichloromethane extract.
The crude extract (100 mg) was dissolved in small amount of solvent and then filtered through Pasteur pipette. The Sep-Pak column was washed with 100% MeOH and then equilibrated with water. The solvent then was allowed to evaporate. Sample was injected to the column through injector. It was then washed gradiently with each 3 mL of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100 % MeOH respectively with flow rate 3mL/min. Each fraction was collected, evaporated and subjected to bioassay. The most active fraction, F9, fraction from 80% MeOH:20% H2O was washed with dichloromethane to give yellow solid.
The solid was recrystallised from chloroform to give a white crystal. The crystal melted at 125-128oC.
The active compound was characterized at Griffith University-Australia, using spectroscopic techniques (IR, NMR and MS). The structure of the compound was elucidated by interpretating the IR spectra, mass spectra, 1HNMR, 13 CNMR, DEPT, HCOSY, HMCOSY and HMBC spectra data (Silverstein, 1991).
Bioassay
Bioassay was carried out using larva Artemia salina L. as bioindicator to monitor toxicity of plant extract during fractionation. The procedure was described as follows: ten shrimps were transferred to each sample vial containing extracts at concentration of 10, 100 and 0 µg/mL (control), using a pasteur pipette. Sea water which has been filtered was then added to the sample vials to make 5 mL. Survivors were counted after 24 hr and percentage of death at each concentration and control were determined. The experiment was done with three replicated.
RESULT AND DISCUSSION
Fractionation and bioassay result are summarized in Table 1.
Table1. Fractionation and bioassay results
|
Frac-tion |
Percentage death of A.s larvae at 24 hr (%) Eluen % MeOH Weigh of fraction (mg) 10 100 ppm ppm |
|
F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 |
0 - - - 10 - - - 20 - - - 30 - - - 40 - - - 50 - - - 60 - - - 70 9.4 0 50 80 10.2 90 100 90 18.5 66 100 100 5.4 0 20 |
- = not determined
The active component of leaves Momordica charantia, L was isolated as a white crystal melted at 125-128oC.
1HNMR (CDCl3): 9.8 (1H,19-H); 5.89 (1H, 6-H); 5.08 (1H, 24-H); 4.38 (1H, 23-H); 3.89 (1H, 7-H); 3.5 (1H, 3-H); 2.4 (1H, 10-H); 2.0 (1H, 8-H); 1.9 (1H, 16-H); 1.6 (1H, 16-H); 1.8 (1H, 2-H); 1.7 (1H,2-H); 1.7 (3H, 27-H); 1.7 (3H, 26-H); 1.7 (2H, 11-H); 1.7 (1H, 20-H); 1.6 (1H, 1-H); 1.5 (1H, 1-H); 1.4 (1H, 17-H); 1.2 (3H, 29-H); 1.2 (2H, 12-H); 1.3 (2H, 15-H); 1.0 (3H, 30-H); 0.9 (2H, 22-H); 0.85 (3H, 21-H); 0.8 (3H, 18-H); 0.7 (3H, 28-H).
13CNMR (CDCl3): 207.8 (CH, 19-C); 145 (C, 5-C); 133 (C, 25-C); 129 (CH, 24-C); 123(CH, 6-C); 76.5 (CH, 3-C); 66 (CH, 23-C); 65.9 (CH, 7-C); 51 (C, 9-C); 50 (CH, 17-C); 49 (CH, 8-C); 48 (C,13-C); 46 (C, 14-C); 45 (CH2, 22-C); 42 (C, 4-C); 37 (CH, 10-C); 34 (CH2, 15-C); 33 (CH, 20-C); 30 (CH2, 12-C); ); 29 (CH2, 16-C); 28.5 (CH2, 11-C); 28 (CH3, 29-C); 26 (CH3, 30-C); 25 (CH3, 26-C); 23 (CH2, 1-C); 21(CH2, 2-C); 18.5 (CH3, 28-C); 18 (CH3, 21-C); 17 (CH3,27-C); 15 (CH3, 18-C).
MS: [M+] 472.6, corresponding to C30H46O4.
IR (υ max cm-1): 3418; 2945; 2871; 1708; 1663; 1467; 1382.
The high resolution mass spectrum of the active compound gave a molecular ion peak M+ = 472.6 which was consistent with the molecular formula C30H48O4. The IR spectrum (Silverstein, 1991) suggested the presence of a hydroxyl group (υ max cm-1= 3418), an aldehyde group fuction (υ max cm-1= 1708) and C=C (υmax cm-1 = 1663). The 1H- {and 13C-} spectra showed the presence of an aldehyde group at δ 9.8-{δ 207.8}, three secondary carbinyl group at δ 4.38-{δ 66}; 3.89-{δ 65.9} and 3.5-{δ 76.5}; two trisubstituted double bond at δ 5.89-{δ 123} and 5.08-{129}, and seven methyl group at δ 0.7; 0.8; 0.85; 1.0; 1.2; 1.7 and 1.7. In addition to the above mentioned signals the DEPT spectra showed signals for six quartenary carbon (δ 145; 133; 51; 48; 46; 42), carbon of methine group (δ 207.8; 129; 123; 76.5; 66; 65.9; 50; 49; 37; 33), carbon of seven methylene group (δ 45; 34; 30; 29; 28; 23 and 21) and carbon of seven methyl group (δ 18.5; 15; 18; 28; 26; 17 and 25).
All of the information above led us to suggest that the active compound was momordicin I or 3,7,23-trihydroxycucurbitan-5-
24-dien-19-al. This compound has the same carbon skeleton as glycoside momordicoside isolated from immature fruit of M. charantia (Okabe, 1982)
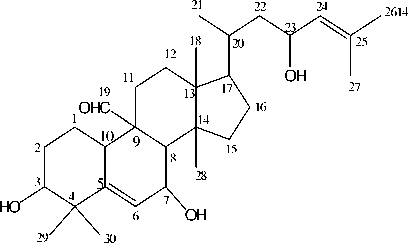
Momordicin I (3,7,23,-Trihydroxycucurbitan-5,24-dien-19-al
The structure was confirmed by the spectral data compared of the same compound from publication (Yasuda, 1984, Fatope, 1990).
CONCLUSION
From this research, momordicin I or 3,7,23-trihydroxycucurbitan-5-24-dien-19-al was isolated from the leaves extract of Momordica charantia, L.
ACKNOWLEDGEMENT
The author is very grateful to Associate Prof. David Young and Dr. Tony from Griffith University for the facility, support and assistance given in characterization of the compound. Thanks also to IAEUP-DIKTI for the financial support.
REFERENCES
Ali, L., Azad Khan, A. K., Mumun, M. I. R., Mosihuzzaman, M., Nur-e-Alam, M, and Rokeya, B., 1993, Studies on
Hypoglycemic Effects of Fruit Pulp, Seed, and Whole of Momordica
charantia on Normal and Diabetic Model Rats, Planta.Med,53 : 409-412
Begum, S., Ahmed, S., Siddique, B. S. M Khan, A., Safy, Z. S. and Arif, M, 1997, Triterpenes, A Sterol and A Monocyclic Alcohol from Momordica charantia, Phytochemistry; Elsevier Science Ltd, 44 (7) : 1313-1320
Fatope, M. O., Takeda, Y., Yamashita, H., Okabe, H., and Yamauchi, T., 1990, New Cucurbitane Triterpenoid from Momordica, J. Nat. Prod, 53 (6) : 14911497
Hamato, N., Koshiba, T., Pham, T. N., and Tatsumi, Y., 1995, Tripsin and Elastase Inhibitors From Bitter Gourd (Momordica charantia Linn) Seeds: Purification, Amino Acid Sequences, and Inhibitory Activities of Four New Inhibitors, J. Biochem, 117 : 432-437
Meyer, B. N., Ferrigni, N.R and McLaughlin, 1982, Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents, Planta Medica, 45: 31-34.
NG, T. B., N., Liu, W. K., Sze, S. F., and Yeung, H. W., 1994, Action of α-Momorcharin, a Ribosome Inactivating Protein, on Cultured Tumor Cell Lines, Gen Pharmac, 25 (1) : 75-77.
Okabe, H., Miyahara, Y., Yamauci, T., 1982, Studies on the Constituents of Momordica charantia L
Chem.Pharm.Bull., 30 (12) : 4334-4340
Puspawati, N. M., Tengah, I G. P., dan Mulyono, 1997, Extractions and Characterisation of Biologically Active Compounds from Balinese Plants Used in Traditional Medicines, Research Report, Universitas Udayana, Denpasar-Bali.
Silverstein, R. M., 1991, Spectrometric
Identification of Organic Compound, 5th ed, John Wiley & Sons, INC, Singapore.
Yasuda, M., Iwamoto, M., Okabe, H and Yamauchi, T., 1984, A New Cucurbitane Triterpenoid From Momordica charantia, Chem, Pharm, Bull, 32 (6) : 2044-2049
56
Discussion and feedback