Callus Induction In Leucaena (Leucaena leucocephala (Lam.) de Wit) As An Effort To Provide Target Transformation Through Agrobacterium tumefaciens
on
Callus Induction in Leucaena (Leucaena Leucocephala (Lam.) De Wit) as an Effort to Provide Target Transformation
Through Agrobacterium Tumefaciens
Arieswari, NNN, Astarini, IA, Hardini, J, Garrido, AR, Margareth, D, Crismonika, J, and Cocioba, SS.
CALLUS INDUCTION IN LEUCAENA (Leucaena leucocephala (Lam.) de Wit) AS AN EFFORT TO PROVIDE TARGET TRANSFORMATION THROUGH Agrobacterium tumefaciens
Ni Nyoman Nila Arieswari1*, Ida Ayu Astarini1, Junita Hardini1, Austin Ryan Garrido2, Debora Margareth2, Jennifer Crismonika2, and Sebastian S. Cocioba3 1Master Program in Biological Sciences, Faculty of Mathematics and Natural Sciences, Udayana University, Bali, Indonesia 2PT. Kultiva Life Sciences, Bali, Indonesia
3Binomica Labs, New York City, United States of America
*Corresponding author: nilarieswari@gmail.com
ABSTRACT
Received:
7 August 2022
Accepted:
25 September 2022
Published:
30 September 2022
INTRODUCTION
Leucaena is a plant that produces biomass productivity in the form of hardwood for fuel. However, Leucaena is also classified as an invasive plant which can cause the urgency of native plant species and ecosystems in Indonesia. Therefore, the formation of sterile Leucaena needs to be done, one of which is through genetic transformation using Agrobacterium tumefaciens. Callus is used as a target for transformants in the genetic transformation process, so it is necessary to use appropriate media and PGR. This study aimed to determine the type of media and the concentration of 2.4-D on callus induction. This research is an experimental study with a completely randomized design (CRD) method with two factors. The first factor is the type of media (MS and WPM) and the second factor is the concentration of 2.4-D (0; 0.25; 0.50; 0.75; 1.00; 1.25 and 1.50 mgL-1 ). Parameters observed were callus initiation, callus fresh weight (gram), callus texture and color. Quantitative data is analyzed by analysis of variance (ANOVA). The results showed that the use of media had a significant effect (P<0.05) on callus fresh weight. The use of 2,4- D concentration had a significant effect (P>0.05) on callus texture. The use of WPM media resulted in the fastest callus emergence time (6.67±0.57), the best callus texture (crumb callus type 2) and the best callus color (green). Meanwhile, the highest fresh weight (2.48±0.83) was in the use of MS media. The fastest callus emergence time occurred in the control (without the addition of 2.4-D) (7.33±0.57 and 6.67±0.57), the highest average fresh callus weight (2.48±0.83 and 2.35±0.32) occurred in the treatment with the addition of 1.00 mgL-1 2.4-D with a crumb callus texture of type 2 and 0.25 mgL-1 2.4-D produced callus with green color.
Keywords: 2.4-D, Genetic Transformation, Lamtoro, MS, WPM
‘Lamtoro’ in Indonesia and is the finest
Leucaena leucocephala (Lam.) de
Wit (Fabaceae), commonly known as
known species of the genus Leucaena. Leucaena is a fast-growing, nitrogen-fixing
leguminous tree that develops in a wide extend of soil sorts in numerous tropical and sub-tropical nations (Saafi and Borthakur, 2002; Jube and Borthakur, 2008). It is considered an indispensable tree species in agroforestry because of its disease resistance, efficient development and its branches can be used as food for feeding organisms. Leucaena leaves are high in protein, but they also contain a toxic called mimosine (Saafi and Borthakur, 2002).
Leucaena has a high biomass, but this plant is classified as an invasive plant. Invasive plants originating from within the country and abroad can cause the urgency of native species and ecosystems (Radiansyah et al., 2015). Leucaena was declared as an alien invasive plant in Indonesia with a higher density of tiller growth (11.78%) in uninhabited houses and 4.42% in inhabited houses (Iqbar et al., 2017). It is also declared as invasive plants along the hiking trail of Panderman Mount, East Java. This plant can grow at an altitude of more than 1400 meters above sea level (masl) and is able to withstand rainfall of 500-3500 mm (Septiadi et al., 2018). One of the reasons is the easy spread of Leucaena seeds, either through biotic or abiotic aids. However, even without apparent fruit or seed morphological adaptation, endozoochory can be an effective mechanism for an alien species to spread to
eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2022.v10.i01.p02
a new environment, even if it did not spread in this way within its home range (Gallego-Fernandez et al., 2020).
Prevention needs to be done on the invasive nature of Leucaena without reducing its biomass productivity through the formation of sterile Leucaena. Research on the manufacture of sterile Leucaena has been carried out, using the EMS mutagen (Ethyl Metane Sulfonate) in which 27 of the 179 plants that experienced mutagenesis were considered sterile (Mcmilan et al., 2019). Interspecific hybridization in Leucaena plants has also been carried out and resulted in sterile triploid Leucaena plants (Sorensson and Brewbaker, 1994). Another method that can be done to produce sterile Leucaena is by genetic transformation.
Hereditary change utilizing
Agrobacterium tumefaciens or the biolistic strategy requires a productive tissue culture recovery convention. Tissue culture work of this tree has been started in a number of research facilities with constrained victory (Saafi and Borthakur, 2002). The initial stage of the genetic transformation process is callus production in vitro (Dwiyani et al., 2016). Callus is a transformation target which will be inoculated with A. tumefaciens suspension in the genetic transformation process. The use of callus as a target for transformation has been carried
out on various plants. Correia (2014) used callus as a transformation target in the manufacture of Leucaena plants with low phenolic exudate, low cell necrosis and a stronger root system mediated by A. tumefaciens.
Research on callus induction of Leucaena plants generally uses MS media (Sapsuha et al., 2011; Manpaki et al., 2018; Prihantoro et al., 2019). The research of Gautam and Gautam (2020) compared the use of three media in the propagation of Leucaena plants, namely MS, NB and B5 media. The results showed that callus could be induced with B5 (13%), MS (6%) and NB (0%) media. However, the use of WPM media for woody plants needs to be considered. Arieswari et al. (2018) produced the highest callus induction with WPM media without ZPT (60%) on grapes. Preliminary tests have also shown that the fastest callus induction (7 days after induction) was produced using WPM media.
Plant growth regulator (PGR) concentration was the main factor
MATERIALS AND METHODS Materials
The materials used were Leucaena seeds cv. Tarramba (brown color), MS media, WPM media, ZPT stock solution
controlling callus formation in culture media. PGR concentrations may vary for each plant species and may even depend on the explant source or individual plant genotype, age, nutritional status and others. 2.4-D is the most commonly used auxin to induce callus formation (Smith, 2013).
Research conducted by Sapsuha et al. (2011) showed that the optimal treatment to induce callus in Leucaena plants was obtained at a concentration of 4 mgL-1 2.4-D. The optimal combination of NAA and kinetin phytohormones in the formation of somatic embryos is 1.5 mgL-1 NAA and 2.0 mgL-1 kinetin. Meanwhile, Prihantoro et al. (2019) reported that cytokinin Thidiazuron (TDZ) 0.5 ppm gave the best callus diameter response and was able to produce a crumb callus texture, but showed a callus color response that tended to be light green.
The aim of this experiment was to determine the best medium (between MS and WPM) and the best concentration of 2.4-D in inducing Leucaena callus to be used as transformation targets.
-
2.4 -D 1000 mgL-1, sucrose, agar, sterile distilled water, 70% and 95% alcohol, sterilant (detergent, fungicide (Dithane), bactericide, bleach 5% and bleach 10%) and warm water.
Tools
The tools used were Laminar Air Flow Cabinet (LAF), analytical balance, Beaker glass, Erlenmeyer flask, Petri dish, bottles, measuring cylinder, forcep, scalpel and blade, micro pipette, spoon, hot plate stirrer, pH meter and autoclave.
Methods
Leucaena seeds were obtained from Sumurgeneng Village, Jenu Sub-district, Tuban Regency, East Java. In vitro culture experiment was carried out at Plant Tissue Culture Laboratory, PT. Kultiva Life Sciences, Lodtunduh, Ubud Subdistrict, Gianyar Regency, Bali, Indonesia, from December 2021 to April 2022.
Seed Sterilization
The sterilization of Leucaena seeds followed the method of Sapsuha et al. (2011) with modifications. Leucaena seeds were washed with 0.5 g of detergent for 15 minutes then rinsed with sterile water for 10 minutes. Seeds were then soaked in 1% fungicide solution and 1% bactericide solution for 20 minutes each. Then, the seeds were rinsed again with sterile water. The seeds were soaked in a 10% bleach and 5% bleach solution for 10 minutes each and rinsed again with sterile water 3 times for 5 minutes each. Finally, to break dormancy, the seeds were soaked in warm water for 30 minutes in LAF. The sterile seeds were cultured under aseptic conditions in LAF.
eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2022.v10.i01.p02
Seeds were cultured in preconditioned media and kept in a culture room at 250C under 12 watt LED lighting (18 hours light and 6 hours dark) for 14 days to induce shoots growth.
Callus Initiation
The young stem explants were cut into 0.5-1.0 cm pieces and cultured on MS and WPM medium supplemented with 30 gL-1 sucrose and 8 gL-1 agar. The experiment employed Randomized Completely Design with two factors. First factor was basal medium used (MS and WPM). Second factor was 2.4-D concentration (0; 0.25; 0.50; 0.75; 1.0; 1.25 and 1.50 mgL-1). Each treatment was repeated three times and one explant was planted in one Petri dish. Based on that, there were 42 experimental units, namely 2 types of media × 7 concentration of 2.4-D × 3 replications. Cultures were incubated at 250C under white fluorescent tubes at 18 hour light and 6 hour dark. Variables observed were time of callus initiation, callus fresh weight, callus texture and color were measured following callus scoring of Gultom et al. (2012) and Kadir (2006).
Data Analyses
Statistical tests used in this study were normality test, homogeneity test, ANOVA test and Tukey HSD test using IBM SPSS Statistics 24 software.
RESULT AND DISCUSSION
Time of Callus Initiation
In this experiment, 100% of the explant successfully produced callus within 6-13 days after culture (DAC). Callus initiation was fastest on WPM media
without 2.4-D (6.67±0.57) and MS media without 2.4-D (7.33±0.57). The longest time was at the treatment of 1.50 mgL-1 2.4-D (13.33±0.57). The results of the ANOVA test on the time of callus initiation are presented in Table 1.
Table 1. ANOVA test results of Leucaena callus initiation time
|
Concentration of 2.4-D (mgL-1) |
Time of Callus Initiation (Mean ± Std. Deviation) | |
|
MS |
WPM | |
|
0 |
7.33±0.57a |
6.67±0.57a |
|
0.25 |
12.00±1.00cde |
10.00±1.00b |
|
0.50 |
11.67±0.57bcde |
11.33±1.54bcd |
|
0.75 |
12.33±0.57cde |
11.67±1.52bcde |
|
1.00 |
12.00±0.00cde |
11.00±1.73bc |
|
1.25 |
13.00±1.00de |
11.33±0.57bcd |
|
1.50 |
13.33±0.57e |
12.00±1.00cde |
Note: The mean value ± SD followed by different letters in the same column indicate significant difference according to Duncan’s Multiple Range Test (DMRT) at a significance level α = 0.05.
Callus initiation time is affected by PGR combination, medium and plant species used. Callus induction is one of the important steps in genotype selection for tissue culture-based research and plant breeding, especially genetic transformation (Umami et al., 2016). Callus is formed in response to injury and growth regulators in the media (Sapsuha et al., 2011). Mahadi et al. (2016) in his research on callus induction of Kasturi orange (Citrus microcarpa) using 2.4-D and BAP with an in vitro method explained that higher PGR concentration in the growing media resulted
in higher callus growth rate. This is different from the results of this research in which the callus produced varied, namely not only growing on media with PGR but also growing on media without PGR. Lara et al. (2022) also described the same thing where in practice, it mostly depends on the species and the variation of endogenous hormones in the species. This research shows that the use of WPM medium can produce fastest callus. The use of WPM media also resulted in the fastest callus emergence time on Barringtonia racemosa plants (about 7 DAC) compared to MS
media (Osman et al., 2016). The advantages of WPM media are in producing an earlier callus induction response and it takes 3 weeks to produce callus on B. racemosa leaf explants compared to other media which takes 5 weeks (Behbahani et al., 2011).
Callus fresh weight
Growth is a permanent increase in the size of an organism or part of a plant which is the result of an increase in the number and size of cells. Growth is
eISSN: 2655-9994 pISSN: 2303-3371 https://doi.org/10.24843/IJBB.2022.v10.i01.p02
characterized by an irreversible increase in weight, so the measurement of callus fresh weight can represent the callus growth variable (Indah and Ermavitalini, 2013). Fresh weight of callus in this study was determined by looking for the difference between callus weight at week 4 after induction and the first day of culture. The callus fresh weight yield data are presented in Table 2.
Table 2. ANOVA test results of Leucaena callus fresh weight
|
Concentration of 2.4-D (mgL-1) |
Callus fresh weight (Mean ± Std. Deviation) | |
|
MS |
WPM | |
|
0 |
0.80±0.37a |
0.73±0.23a |
|
0.25 |
2.30±0.33bcd |
1.17±0.64abc |
|
0.50 |
1.48±0.48abcd |
1.45±0.27abcd |
|
0.75 |
1.73±0.78abcd |
1.70±1.27abcd |
|
1.00 |
2.48±0.83d |
2.35±0.33cd |
|
1.25 |
1.03±0.69ab |
0.54±0.61a |
|
1.50 |
1.22±0.76abcd |
1.10±0.77abc |
Note: The mean value ± SD followed by different letters in the same column indicate significant difference according to Duncan’s Multiple Range Test (DMRT) at a significance level α = 0.05.
Based on Table 2, WPM media with a concentration of 2.4-D 1.25 mgL-1 (0.54±0.61) did not show a significantly different effect with MS media and WPM media (0.80±0.37 and 0.73±0.23). MS medium with a concentration of 2.4-D 1.0 mgL-1 produced the highest callus weight 2.48± 0.83. MS media with concentrations of 2.4-D 0.50 mgL-1, 0.75 mgL-1 and 1.50 mgL-1 showed results that were not
significantly different from WPM media 0.50 mgL-1 and 0.75 mgL-1.
Irreversible weight gain characterizes growth, so the fresh weight measurement of callus can represent callus growth variables (Sari et al., 2018). The fresh weight produced is very dependent on the speed at which these cells divide, multiply, and then continue to grow (Idris and Paserang, 2019). According to Marisa et al. (2021), physiologically fresh weight
consists of water and carbohydrates. Indah and Ermavitalini (2013) described that the large fresh weight of callus was due to the high water content. Rahayu et al. (2016) showed that the fresh weight of callus tended to increase at a concentration of 2 to 4 mgL-1 2.4-D but decreased at a concentration of 6 mgL-1 2.4-D in Centella asiatica plants.
Callus texture and color
Explant growth in vitro culture could be observed from its visual appearance in the form of callus texture and color. Callus texture is one of the markers used to assess callus quality (Indah and Ermavitalini, 2013). Observation of callus texture was carried out at 4 week after culture which resulted in varied callus textures (Figure 1). The results of the ANOVA test on the texture of the Leucaena callus are presented in Table 3.
Table 3. ANOVA test results of Leucaena callus texture
|
Media |
Concentration of 2.4-D (mgL-1) |
Mean±Standard Deviation |
Note |
|
0 |
3.00±0.00bcd |
Nodular compact callus | |
|
0.25 |
1.67±1.15ab |
Friable callus type 1 | |
|
0.50 |
2.33±1.15abcd |
Nodular compact callus | |
|
MS |
0.75 |
2.33±0.57abcd |
Nodular compact callus |
|
1.00 |
1.00±0.00a |
Friable callus type 1 | |
|
1.25 |
2.00±1.73abc |
Friable callus type 1 | |
|
1.50 |
3.33±0.57bcd |
Nodular compact callus | |
|
0 |
3.00±0.00bcd |
Nodular compact callus | |
|
0.25 |
3.00±0.00bcd |
Nodular compact callus | |
|
0.50 |
3.00±0.00bcd |
Nodular compact callus | |
|
WPM |
0.75 |
2.67±0.57bcd |
Nodular compact callus |
|
1.00 |
2.67±0.57d |
Friable callus type 2 | |
|
1.25 |
3.00±0.00bcd |
Nodular compact callus | |
|
1.50 |
2.67±0.57bcd |
Nodular compact callus |
Note: The mean value ± SD followed by different letters in the same column indicate significant difference according to Duncan’s Multiple Range Test (DMRT) at a significance level α = 0.05.
The results showed that WPM treatment with a concentration of 2.4-D 1.0 mgL-1 gave a significantly different effect on callus texture compared to other treatments. Meanwhile, MS treatment without 2.4-D and WPM without 2.4-D did
not show a significantly different effect with MS treatment with a concentration of 2.4-D 1.50 mgL-1, WPM with a concentration of 2.4-D 0.25 mgL-1, WPM with a concentration of 2.4-D 0.50 mgL-1, WPM with a concentration of 2.4-D 0.75
mgL-1, WPM with a concentration of 2.4-D
-
1.25 mgL-1 and WPM with a concentration
of 2.4-D 1.50 mgL-1.
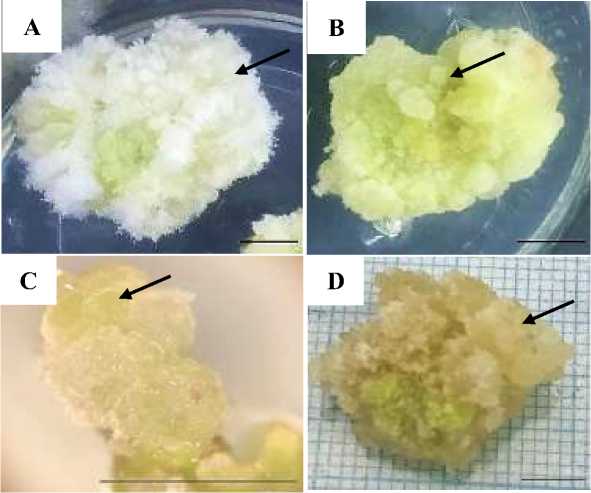
Figure 1. Leucaena callus texture. A. Friable callus type 1. B. Compact callus. C. Nodular compact callus. D. Friable callus type 2. The black arrow indicates the callus texture type. Magnification C: 20X. Bars: 0.5 cm.
Callus texture is a marker used to determine the quality of a callus so that it can be seen that cells are still actively dividing or have stagnated in cell division. A good callus is assumed to have a friable texture because it facilitates separation into single cells and increases oxygen aeration between cells (Sari et al., 2014). Compact callus is more difficult to separate because of its strong texture. However, compact callus usually produces high secondary metabolites which are commonly used in the health sector (Arieswari, 2018).
Callus color is one of the indicators of explant growth on in vitro culture. Callus
tissue produced from an explant usually brings out different colors (Indah and Ermavitalini, 2013). Callus color observations were carried out at 4 week after culture (Figure 2). The results of the ANOVA test on the color of the Leucaena callus are presented in Table 4.
Based on Table 4, WPM treatment with a concentration of 2.4-D 0.25 mgL-1 showed a significantly different effect compared to other treatments. This treatment produces a green callus. MS treatment without 2.4-D and WPM without 2.4-D gave no significant effect with WPM treatment with a concentration of 2.4-D
0.50 mgL-1 and WPM with a concentration
of 2.4-D 0.75 mgL-1, which resulted in
blackish-brown callus.
Table 4. ANOVA test results of Leucaena callus color
|
Media |
Concentration of 2.4-D (mgL-1) |
Mean±Standard Deviation |
Note |
|
0 |
2.00±1.00a |
Blackish-brown | |
|
0.25 |
5.00±1.00c |
White | |
|
0.50 |
3.67±1.58abc |
Yellowish white | |
|
MS |
0.75 |
3.67±2.08abc |
Brownish yellow |
|
1.00 |
3.00±1.00ab |
Brownish yellow | |
|
1.25 |
5.33±0.57cd |
Whitish green | |
|
1.50 |
5.33±1.15cd |
Whitish green | |
|
0 |
2.00±0.00a |
Blackish-brown | |
|
0.25 |
7.00±0.00d |
Green | |
|
0.50 |
2.33±0.57a |
Blackish-brown | |
|
WPM |
0.75 |
2.33±0.57a |
Blackish-brown |
|
1.00 |
2.67±0.57ab |
Brownish yellow | |
|
1.25 |
2.67±0.57ab |
Brownish yellow | |
|
1.50 |
4.33±1.15cd |
White |
Note: The mean value ± SD followed by different letters in the same column indicate significant difference according to Duncan’s Multiple Range Test (DMRT) at a significance level α = 0.05.
Other treatments such as MS with a concentration of 2.4-D 1.25 mgL-1 and MS with a concentration of 2.4-D 1.50 mgL-1 which was whitish green showed no significant effect on WPM with a concentration of 2.4-D 1.50 mgL-1 which is white. MS treatment with a concentration of 2.4-D 0.75 mgL-1 and MS with a concentration of 2.4-D 1.0 mgL-1 showed no significant effect on WPM with a concentration of 2.4-D 1.0 mgL-1 and WPM with a concentration of 2.4-D 1.25 mgL-1, ie brownish yellow. Meanwhile, yellowish white color was only produced in MS
treatment with a concentration of 2.4-D 0.50 mgL-1.
Callus color indicates cell division activity that occurs in callus (Damanik et al., 2018). Green, white, yellow and brown colors indicate that cells are still actively dividing, while brown, black or blackish brown colors indicate signs of cell aging. Callus discoloration is caused by the synthesis of phenolic substances in cells (calus) (Nasution and Nasution, 2019).
The brown color of the callus is caused by the metabolism of toxic phenolic compounds, and often occurs as a result of
the explant sterilization process. Phenol compounds generally inhibit growth or even cause tissue death (Nasution and Nasution, 2019). I’anatushshoimah, et al. (2020) stated that the browning event is
actually a natural event and a process of adaptive change of plant parts due to physical influences such as stripping and cutting.
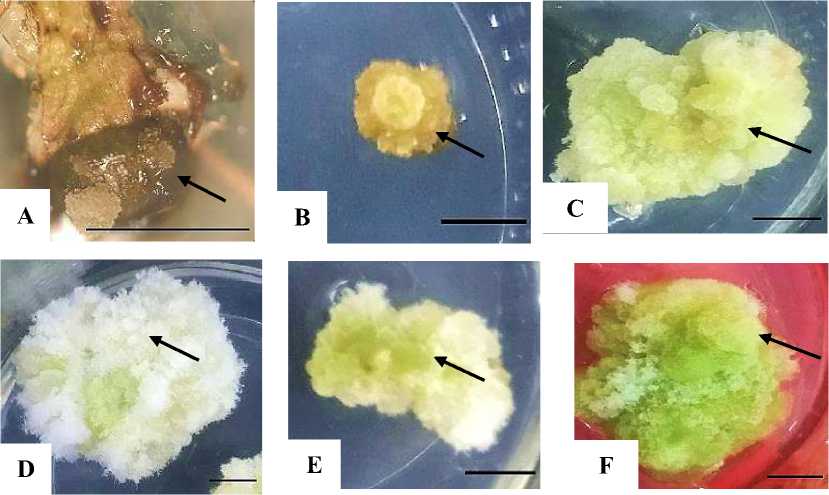
Figure 2. Leucaena callus color. A. Blackish-brown. B. Brownish yellow. C. Yellowish white. D. White. E. Whitish green. F. Green. The black arrow indicates the callus color. Magnification C: 20X. Bars: 0.5 cm.
In another study conducted by Gautam and Gautam (2014), B5 media with 0.21 mgL-1 2.4-D was able to produce Leucaena callus production reaching 100%. Manpaki et al. (2018) showed that the use of 2.4-D with concentrations of 0.5, 1.0, 1.5 and 2.0 mgL-1 resulted in a compact callus texture.
CONCLUSIONS
The use of WPM without 2.4-D can induced fastest time of callus initiation, WPM with 2.4-D 1.0 mgL-1 can produced
Prihantoro (2019) using other hormones, namely BAP and TDZ, showed that 0.5 ppm TDZ gave the best callus diameter response and was able to produce a crumbly callus texture, but showed a callus color response that tends to be light green.
friable callus type 2 and also WPM with 2.4-D 0.25 mgL-1 can produced green color callus. However, the use of MS with 2.4-D
-
1 .0 mgL-1 can induced the highest fresh weight callus.
ACKNOWLEDGMENT
The authors gratefully
acknowledged support of Kultiva Life Sciences for the provision of all the materials, tools, laboratory and support to do this research. Thanks also to Dr. Dra. Eniek Kriswiyanti, M.Si., Ir. Made Pharmawati, M.Sc., PhD and Dr. Ir. Made Ria Defiani, M.Sc. (Hons) for constructive criticism.
REFERENCE
Arieswari, N. N. N., Astarini, I. A., Astiti, N. P. A., & Pramana, J. (2018). In vitro callus induction of ‘shiraz’ grape (Vitis vinifera L.) using different medium and growth regulator combination.
International Journal of
Biosciences and Biotechnology, 6(1), 25-33.
https://doi.org/10.24843/IJBB.2018 .v06.i01.p04.
Behbahani, M., Shanehsazzadeh, M., & Hessami, M. J. (2011). Optimization of callus and cell suspension cultures of Barringtonia racemosa (Lecythidaceae family) for Lycopene Production. Sci. Agric., 68, 69–76.
https://doi.org/10.1590/S0103-90162011000100011.
Correia, J. (2014). Enhancement of Leucaena leucocephala Tissue Regeneration and Agrobacterium-Mediated Transformation (Thesis). Hawai: University of Hawai’I at Manoa.
Damanik, R. I., Manurung, B. H., & Bayu, E. S. (2018). Effects of hypoxia condition in embryogenic callus growth of soybean cell culture. International Conference on Agriculture, Environment, and Food Security, 122: 012056.
Dwiyani, R., Yuswanti, H., Darmawati, I.A.P., & Mayadewi, N.N.A.
-
(2016) . transformasi genetik pada tanaman melalui Agrobacterium tumefaciens. Denpasar: Swasta
Nulus.
Gallego-Fernandez, J. B., Martinez, M. L., Garcia-Franco, J. G., &
Zunzunegui, M. (2020). Multiple seeds dispersal modes of an invasive plant species on coastal dunes. Biol. Invasions, 23, 111-127.
https://doi.org/10.1007/s10530-020-02359-6.
Gautam, V. K. & Gautam, N. (2014). In vitro differentiation from cultured explants of Leucaena leucocephala. Research & Reviews in Biotechnology & Biosciences, 1(1), 57-61.
Gautam V. K., & Gautam, N. (2020).
Micropropagation of Leucaena leucocephala from in vitro cultured shoot tip explants. International Journal of Bioinformatics and Biological Sciences, 8(2), 11-14.
https://doi.org/ 10.30954/2319
5169.2.2020.3.
Gultom, M. S., Anna, N., & Siregar, E. B. M. (2012). Respon eksplan biji gaharu (Aquilaria malaccensis
Lank.) terhadap pemberian IAA secara in vitro. Peronema Forestry Science Journal, (1), 1-6.
I’anatushshoimah, Nurcahyati, Y., Prihastantii, E. & Hastuti, R. B. (2020). Effects of light for callus
induction of mangrove plant (Rhizophora apiculata Bi) by in vitro. Life Science, 9(2), 138-148. https://doi.org/10.15294/lifesci.v9i 2.47157.
Idris, S. R. L. R., & Paserang, A. P. (2019). Induksi kalus tanaman kentang dombu (Solanum tuberosum L.) secara in vitro dengan pemberian ZPT 2.4-D (Dichlorophenoxy Acetid Acid). Natural Science: Journal of Science and Technology, 8(2), 110-115.
https://doi.org/10.22487/25411969. 2019.v8.i2.13538.
Indah, P. N., & Ermavitalini, D. (2013). Induksi kalus daun nyamplung (Calophyllum inophyllum Linn.) pada beberapa kombinasi
konsentrasi 6-Benzylaminopurine (BAP) dan 2,4-
Dichlorophenoxyacetic Acid (2.4-D). Jurnal Sains dan Seni Pomits, 2(1): 2337-3520.
https://doi.org/10.12962/j23373520 .v2i1.2571.
Iqbar, Riana, S., & Masykur. (2017).
Inventory of the potential invasive plant species in housing area of PT. Arun NGL, Lhokseumawe, Aceh. BioLeuser, 1(1): 20-30.
https://doi.org/10.24815/jn.v16i1.4 359.
Jube, S., & Borthakur, D. (2009).
Development of an Agrobacterium-mediated transformation protocol for the tree-legume Leucaena leucocephala using immature zygotic embryos. Plant Cell Tiss. Organ Cult., 96, 325-333.
https://doi.org/10.1007/s11240-008-9490-x.
Kadir, A. (2006). Induksi dan perbanyakan populasi kalus, regenerasi tanaman serta uji respon kalus terhadap konsentrasi PEG dan dosis iradiasi sinar gamma (skripsi). Makasar: Fakultas Pertanian Universitas Islam Makasar.
Lara, E. Y. C., Imakawa, A. M., da Silva, D., & Sampaio, P. T. B. (2022). In vitro callus induction from different explants of Senna alata (L.) Robx. (fabaceae). Adv. For. Sci,
Mahadi, I., Syafi’I, W., & Sari, Y. (2016). Callus induction of Calamansi
(Citrus microcarpa) using 2.4-D and BAP hormones by in vitro
methodsJurnal Ilmu Pertanian
Indonesia, 21(2): 84-89.
https://doi.org/10.18343/jipi.21.2.8 4.
Manpaki, S. J., Prihantoro, I., & Karti, P. D. M. H. (2018). Growth responses of leucaena embryogenic callus on embryo age differences and auxin 2.4-Dichlorophenoxyacetic acid. JITV, 23(2), 95-102.
https://doi.org/10.14334/jitv.v23i2. 1538.
Marisa, F., Hidayati, L., Sasongko, A. B., & Nuringtyas, T. R. (2021). Callus induction from cotyledon of Gyrinops versteegii (Gilg.) Domke. Jurnal Biologi Tropis, 21(2), 427433.
http://dx.doi.org/10.29303/jbt.v21i2 .2629.
Mcmillan, H. E., Liu, G., Shelton, H. M., Dalzell, S. A., Godwin, I. A., Gamage, H., Sharman, C., &
Lambrides, C. J. (2019). Sterile leucaena becomes a reality? Tropical Grasslands-Forrajes
Tropicales, 7(2), 74-79.
https://doi.org/10.17138/tgft(7)74-79.
Nasution, N. H., & Nasution, I. W. (2019). The effect of plant growth regulators on callus induction of mangosteen (Garcinia mangostana L.). IOP Conf. Series: Earth and Environmental Science, 305, 1-7.
https://doi.org/10.1088/1755-1315/305/1/012049.
Osman, N. I., Sidik, N. J., & Awal, A. (2016). Effects of variations in culture media and hormonal
treatments upon callus induction potential in endosperm explant of Barringtonia racemosa L. Asian Pacific Journal of Tropical Biomedicine, 6(2), 143-147.
https://doi.org/10.1016/j.apjtb.2015 .10.007.
Prihantoro, I., Anandia, A., Aryanto, A.T., & Karti, P.D.M.H. (2019). The morphological characteristics of adapted lamtoro (Leucaena leucocephala cv Tarramba) pH 3.4 that produced by 40gy gamma ray irradiation based on differences of cytokinins in tissue culture. Pastura, 8(2), 63-68.
https://doi.org/10.24843/Pastura.20 19.v08.i02.
Radiansyah, A. D., Susmianto, A.,
Siswanto, W., Tjitrosoedirdjo, S., Djoho, D. J., Setyawati, T.,
Sugianti, B., Ervandiari, I., Harmono, S., Fauziah, Alaydrus, R., Arta, A. P., & Gunadhrama, N. (2015). Strategi Nasional dan Arahan Rencana Aksi Pengelolaan Jenis Asing Invasif di Indonesia. Jakarta: Kementrian Lingkungan Hidup dan Kehutanan.
Rahayu, S., Roostika, I., & Bermawie, N. (2016). The effect of types and concentrations of auxins on callus induction of Centella asiatica. Nusantara Bioscience, 8(2), 283287.
https://doi.org/10.13057/nusbiosci/ n080224.
Saafi, H., & Borthakur, D. (2002). In vitro plantlet regeneration from
cotyledons of the tree-legume Leucaena leucocephala. Plant Growth Regulation, 38, 279-285. https://doi.org/10.1023/A:1021591 212710.
Sapsuha, Y., Soetrisno, D., & Kustantinah. 2011. In vitro callus induction and
somatic embryogenesis of Leucaena leucocephala. Berita Biologi, 10(5), 627-633.
https://doi.org/10.14203/beritabiolo gi.v10i5.1921.
Sari, Y. P., Kusumawati, E., Saleh, C., & Kustiawan, W. (2018). Effect of sucrose and plant growth regulators on callogenesis and preliminary secondary metabolic of different explant Myrmecodia tuberosa. Nusantara Bioscience, 10(3), 183192.
https://doi.org/10.13057/nusbiosci/ n100309.
Septiadi, L., Wahyudi, D., Rachman, R. S., Syafrudin, & Alfaruqi, N. T. S. 2018. The invasive plants species along the hiking track of Mount Panderman Nature Tourism, Batu, East Java. Journal of Indonesian Tourism and Development Studies, 6(1), 55-62.
https://doi.org/10.21776/ub.jitode.2 018.006.01.08.
Smith, R.H. (2013). Plant Tissue Culture: Techniques and Experiments. London: Springer.
Sorensson, C. T., & Brewbaker, J. L.
(1994). Interspecific compatibility among 15 leucaena species leguminosae: mimosoideae) via
artificial hybridizations. American Journal of Botany, 81, 240‒247. https://doi.org/10.1002/j.1537-2197.1994.tb15435.x.
Umami, N., Akashi, R., Gondo, T., Ishigaki, G., & Tanaka, H. (2016). Study on callus induction system of 4 genotype of napier grass
(Pennisetum purpureum). Animal Production, 18(3), 131-140.
https://doi.org/10.20884/1.anprod.2 016.18.3.528.
25
Discussion and feedback