EFFECT OF BACTERIAL VOLATILE COMPOUNDS ON PAKCOY (Brassica rapa L.) GROWTH PROMOTION
on
Effect of Bacterial Volatile Compounds on Pakcoy (Brassica Rapa L.) Growth Promotion
Phabiola, T.A., Khalimi, K. & Wiguna, P.P.K.
EFFECT OF BACTERIAL VOLATILE COMPOUNDS ON PAKCOY (Brassica rapa L.) GROWTH PROMOTION
Trisna Agung Phabiola1*, Khamdan Khalimi1, Putu Perdana Kusuma Wiguna1 1Departement of Agroecotechnology, Faculty of Agriculture,
Udayana University, Bali, Indonesia
*Corresponding author: phabiola@unud.ac.id
ABSTRACT
This research was aimed to test of the ability of MVOC-producing bacteria to increase plant growth of Pakcoy (Brassica rapa L.). The methodology including testing the ability of MVOC-producing rhizobacteria in Pakcoy plant growth enhancement, MVOC extraction and analysis of compounds in MVOC Extracts using Gas Chromatography. Different bacterial species produce different MVOC. S. maltophilia Sg3 emitted 20 MVOC compounds and MVOC that contribute to increasing plant growth, namely oxalic acid, cyclohexyl undecyl ester, 2-Furancarboxaldehyde, 5-methyl-, 1,2 butanediol, and Piperazine. E. asburiae MjSg48 emitted 12 MVOC compounds and those that contributed to increasing plant growth were oxalic acid, cyclohexyl dodecyl ester and 4-methyl oxazole. E. asburiae TK24 emitted 27 MVOC compounds and those that contributed to increasing plant growth were oxalic acid, isohexyl neopentyl ester, thiazole, Oxalic acid, and cyclohexyl decyl ester. Meanwhile P. rettgeri Al2TT emitted 13 MVOC compounds and those that contributed to increasing plant growth were oxalic acid, diisohexyl ester, and Pyridine, 2,3,4,5-tetrahydro.
Keywords: Microbial volatile organic compound (MVOC), growth, pakcoy
INTODUCTION
Rhizobacteria is bacteria that utilize plant root exudate to live as a colony in rhizosphere area. The metabolite products that released by this rhizobacteria is a chemical compound which easily evaporate or frequently categorized as an organic compound with a volatile property that produced by microbes or Microbial volatile organic compound (MVOC) (Kanchiswamy,
et al., 2015, Cappellari, et al., 2020). The microbes other than rhizobacteria, such us yeast dan fungi also can produce MVOC. The research on the ability of microbes in producing organic compounds which easily evaporate has been published in many journal (Ramirez, et al., 2010, Schmidt, et al., 2015, Gupta, et al., 2017) . The fresh smells from the pure culture of microbes like yeast and citric or lactic acid bacteria, and the strong
smells from various fermented products are a simple proof the existence of compounds from organic acid group as citric acid, acetic acid, lactic acid, propionate, alcohol, ester, mercaptan, pentylfuran and others which are metabolic product of microbes. However, scientific evidence on MVOC activity as a growth inducer and plant resistance was only published in 2003 (Ryu et al. 2003; Ryu et al. 2004). Ryu et al. (2004) reported that Bacillus sp. strain GB03 which emitted 2,3 butanediol and acetoin compounds was able to induce the growth of Arabidopsis thaliana plants when compared to water control and treatment with Escherichia coli strain DH5α. Since then, the role of the MVOC compounds group for plant growth inducers has received a lot of attention and has attracted the interest of other researchers to explore and study its potential development and applications.
The exploration and characterization of the biological function of MVOCs can reveal a variety of biological processes that are important for plant growth (Castro et al., 2009, Bohm, et al., 2017). Castro et al., (2009) stated that Acyl homoserine lactone (AHL) is a compound that is emitted by bacterial cells to regulate gene expression when the population has reached a sufficient level of cell density and intermediate chain AHL compounds (C8-C14) such as N-
hexanoylhomoserine. lactone, N-3-oxo-hexanoyl-homoserine lactone, N-octanoyl-homoserine lactone, N-decanoyl-homoserine lactone, N-dodecanone-homoserine lactone, and N-tetradecanoylhomoserine lactone can be recognized by plants and are able to change gene expression in plants roots and shoots so that they are able to modulate plant cell growth and medium chain AHL compounds can change root architecture, alter primary root growth, stimulate lateral root formation, and root hairs development in A. thaliana plants. MVOC emitted by Alternaria alternata can trigger an escalation in starch accumulation in potato leaves accompanied by upregulation of sucrose synthase, invertase inhibitors, and starch synthase classes III and IV, glucose-6-phosphate transporter, plastidial thioredoxin enzyme, starch breakdown enzymes, and proteins involved in the provision of internal amino acids. This phenomenon is called MVOC-ISAP or MVOC-induced starch accumulation process (Ezquer et al., 2010, Orzechowski, et al., 2021). The rhizobacteria Serratia odorifera emitted compounds 2-pentanone, 4-heptanone, 2-heptanol, 2-undecanone, 2tridecanone, 2-pentadecanone, sodorifen, bicyclic oligomethyl octadiene, 1-hexanol and indole (Kai, et al. 2010, Kanchiswamy, et al., 2015). Indole and
pentadecane compounds produced by S. odorifera can stimulate the growth of A. thaliana plants (Blometal, 2011, Park, et al., 2015).
This research is aimed to aimed to test of the ability of MVOC-producing bacteria to increase plant growth of Pakcoy (Brassica
MATERIALS AND METHODS
The research was conducted in Octoer until December 2021 in Laboratory of Plant Pests and Diseases, Faculty of Agriculture, Udayana University and in the
rapa L.). Pakcoy is a type of leafy vegetable crops that are very important in Indonesia because it has a high economic value. Pakcoy much in demand as a vegetable because of high nutrient content and it tastes good (Gustiar, et al., 2022).
greenhouse in the Experimental Garden of the Faculty of Agriculture, Udayana University, Bali Indonesia. Figure 1 shows the map of the research location.
‰∣ya Pt*pυc^
Experimental Garden
S∣d∂∣<∂
Sesetan
Pedungan
Pemogan
⅛⅛'∙
Pemecutan Klod
Laboratory of P
ests and Diseases
Experimental Garden
Panjer
ibian
Dahgin Puri Kiod
Laboratory of Plant Pests and Diseases Dauh Puri Klod
Dauh Purl Kauh f
Dauh Puri ⅛
Figure 1. The Map of the Research Location.
Testing the ability of MVOC-producing rhizobacteria in Brassica rapa L. Plant Growth enhancement.
The ability of MVOC-producing rhizobacteria to increase plant growth was carried out in the Greenhouse. MVOC producing rhizobacteria were grown for 24 hours in Nutrient Broth medium (10 g peptone, 10 g Beef Extract, 5 g Sodium Chloride). Furthermore, the rhizobacteria were cultured in MRVP broth medium (7 g peptone, 5 g dipotassium phosphate, 5 g dextrose) for 48 hours. The seeds of pakcoy were sterilized using 0.5% Clorox and then soaked in sterile water for 24 hours. The seeds were drained and then soaked with rhizobacteria suspension from MRVP broth culture media for 2 hours.
Furthermore, the seeds were planted in pots, where the bottom of the pot contained a suspension of rhizobacteria from MRVP broth culture media. The measurement of chlorophyll levels in the leaves was carried out using the Minolta-SPAD 502 Chlorophyllmeter. Leaf chlorophyll measurements were carried out at the age of 14 DAP, 21 DAP and 28 DAP on the 2nd and 3rd leaves from the shoots.
MVOC Extraction
MVOC was extracted using ethyl acetate, hexane, and methanol. A total of 50
ml of the culture filtrate was centrifuged at 1.610 x g for 30 minutes, the supernatant was separated from the bacterial precipitate, filtered with Millipore filter paper (0.45 mm).
Then it was partitioned with ethyl acetate and methanol three times. MVOC compounds can be extracted by partitioning the supernatant using ethyl acetate. The ethyl acetate fraction was then separated from the methanol fraction, then the three ethyl acetate fractions were combined into the ethyl acetate fraction. Furthermore, the ethyl acetate fraction was evaporated using a Vacuum Rotary Evaporator at a temperature of 40oC. The extract or ethyl acetate fraction was then dissolved with 5 ml of methanol and stored in a refrigerator with minus 20o C temperature.
Analysis of Compounds in MVOC Extracts using Gas Chromatography – Mass Spectroscopy (GC-MS)
The Analysis of compounds that contained in the rhizobacterial filtrate was carried out using Gas Chromatography – Mass Spectroscopy (GC-MS QP2010 Ultra Shimadzu). The eluent used was liquid nitrogen, Wakosil ODS/5C18-200 column, size 4.6 x 200 mm, eluent flow rate of 1 ml/minute, temperature 250°C and detected using UV light at 254 nm. The results of the detection were carried out by matching the
molecular weights and fragmentation patterns of the isolated compounds with the compounds in the GC-MS library. By using
RESULTS AND DISCUSSIONS
Results
The results of the test of the ability of MVOC-producing bacteria to increase plant growth showed that the MVOC emitted by S. maltophilia Sg3, E. asburiae TK24, E. asburiae MjSg48, and P. rettgeri Al2TT bacteria was able to increase the growth of pakcoy plants. Growth parameter values such
GC-MS, the molecular weight and molecular structure of contained compounds in the bacterial filtrate can be known and identified.
as plant height, root fresh and dry weight, leaf area, leaf chlorophyll content, fresh and dry weight of plants treated with MVOC emitted by bacteria were higher than growth parameter values in control plants. Figure 2. shows performance of pakcoy plants treated with MVOC. KT: plants without MVOC (S. maltophilia Sg3 (Sg3); E. asburiae TK24 (TK24); P. rettgeri Al2TT (AL2T); E. asburiae MjSg48 (MjSg)).

Figure 2. Performance of Pakcoy plants treated with MVOC. KT: plants without MVOC (S. maltophilia Sg3 (Sg3); E. asburiae TK24 (TK24); P. rettgeri Al2TT (AL2T); E. asburiae MjSg48 (MjSg)).
The MVOC treatment in plants was able to increase plant height ranging from 21.44% to 33.97%. MVOC bacteria P. rettgeri Al2TT increased plant height by 21.44%, S. maltophilia Sg3 by 26.84%, E. asburiae TK24 by 26.98%, and E. asburiae MjSg48 by 33.97% when compared to the
control (Table 4.1). The MVOC treatment in plants was able to increase the fresh weight of roots ranging from 15.34% to 63.64%. MVOC bacteria P. rettgeri Al2TT increased root fresh weight by 15.34%, S. maltophilia Sg3 by 63.64%, E. asburiae TK24 by 18.18%, and E. asburiae MjSg48 by 59.09%
when compared to the control. MVOC treatment in plants was able to increase root dry weight ranging from 41.67% to 91.67%. MVOC of P. rettgeri Al2TT bacteria increased root dry weight by 41.67%, S. maltophilia Sg3 by 91.67%, E. asburiae
TK24 by 58.33%, and E. asburiae MjSg48 by 83.33% when compared to controls. Table 1. shows the effect of MVOC on plant height, root fresh weight, and root dry weight of pakcoy plants.
Table 1. The effect of MVOC on Plant Height, Root Fresh Weight, and Root Dry Weight of Pakcoy Plants.
|
Treatments |
Plant Height Root Fresh Weight Root Dry weight (cm) (gr) (gr) |
|
Control E. asburiae MjSg48 S. maltophilia Sg3 E. asburiae TK24 P. rettgeri Al2TT |
20,75 a 17,6 a 1,2 a 27,8 d (33.97%) 28.0 c (59.09%) 2,2 c (83.33%) 26,32 c (26.84%) 28,8 c (63.64%) 2,3 c (91.67%) 26,35 c (26.98%) 20,8 b (18.18%) 1,9 b (58.33%) 25,2 b (21.44%) 20,3 b (15.34%) 1,7 b (41.67%) |
The MVOC treatment in plants was able to increase leaf chlorophyll levels ranging from 25.21% to 40.47%. MVOC bacteria P. rettgeri Al2TT increased leaf chlorophyll content by 25.21%, S. maltophilia Sg3 by 40.47%, E. asburiae TK24 by 28.13%, and E. asburiae MjSg48 by 30.41% when compared to the control. (Table 4.2). The MVOC treatment in plants was able to increase the fresh weight of plants ranging from 75.43% to 94.48%. MVOC of P. rettgeri Al2TT bacteria increased plant fresh weight by 79.63%, S. maltophilia Sg3 by 94.48%, E. asburiae TK24 by 75.43%, and E. asburiae MjSg48 by 87.25% when compared
to controls. Table 2 show the effect of MVOC on leaf chlorophyll content, plant fresh weight, and plant dry weight pakcoy plants.
The MVOC treatment in plants was able to increase the dry weight of the plants ranging from 14.67% to 48.00%. MVOC of P. rettgeri Al2TT bacteria increased plant dry weight by 14.67%, S. maltophilia Sg3 by 48.00%, E. asburiae TK24 by 34.67%, and E. asburiae MjSg48 by 41.33% when compared to controls. The results of MVOC compounds analysis in S. maltophilia Sg3 using GC-MS showed that there were 20 compounds detected shows in Figure 3.
Table 2. The effect of MVOC on Leaf Chlorophyll Content, Plant Fresh Weight, and Plant Dry Weight of Pakcoy Plant.
|
Treatments |
Leaf Chlorophyll Plant Fresh Weight Plant Dry Weight Content (gr) (gr) (SPAD unit) |
|
Control E.asburiae MjSg48 S. maltophilia Sg3 E. asburiae TK24 P. rettgeri Al2TT |
41,16 a 152,2 a 7,5 a 53,68 c (30.41%) 285,0 d (87.25%) 10,6 c (41.33%) 57,82 d (40.47%) 296,0 e (94.48%) 11,1 d (48.00%) 52,74 b (28.13%) 267,0 b (75.43%) 10,1 c (34.67%) 51,54 b (25.21%) 273,4 c (79.63%) 8,6 b (14.67%) |
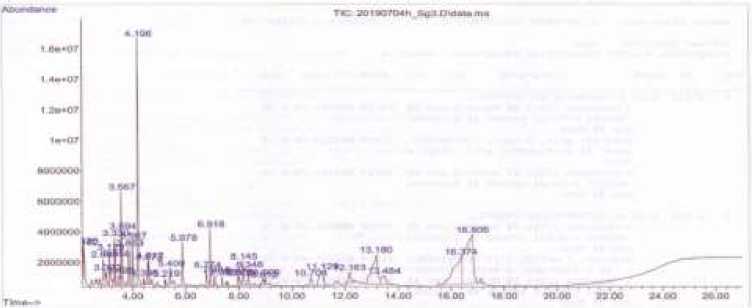
Figure 3. Chromatogram of mVOC in S. maltophilia Sg3
The MVOC compounds in S. maltophilia Sg3 are Cyclobutanol, 1-(trans-2-Phenylcyclopropyl)-2-methylpropan-1-ol, 1-Penthyl-3,3-D2 Acetate, Cyclopropane carboxylic acid, 2(3H)-Furanone, dihydro-, Oxalic acid, cyclohexyl undecyl ester, 7-oxabicyclo[4.1.0] heptane, 1-methyl-, 2-Furancarboxaldehyde, 5-methyl-, 1,3,5-Triazine-2,4,6-triamine, 1 ,4-Dioxan-2-one, 2-hydroxy-butanediol, N-(methyl-d3)
pyrrole, Piperazine, 2-
Furancarboxaldehyde,5-(hydroxymethyl)-, ,2,3-Propanetriol, monoacetate, N,N' –
Diethyl oxamide, n-Pentanal, Tetrahydro-4H-Pyran-4-ol, 1-Pentanamine, N-methyl-N-(1-methylethyl)-, 1,3-Dioxan-5-ol.
The results showed that the MVOC which emitted by S. maltophilia Sg3 and contributed to increasing plant growth were oxalic acid, cyclohexyl undecyl ester, 2-Furancarboxaldehyde, 5-methyl-, 1,2 butanediol, and Piperazine. The results of the analysis of MVOC compounds in E. asburiae MjSg48 using GC-MS showed that there were 12 compounds detected. Figure 4.
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY ∙ Vol. 9 No. 2 ∙ April 2022 eISSN: 2655-9994 pISSN: 2303-3371
https://doi.org/10.24843/IJBB.2022.v09.i02.p05 shows chromatogram of MVOC in E.
asburiae MjSg48.

Figure 4. Chromatogram of MVOC in E. asburiae MjSg48
The MVOC compounds detected in E. asburiae MjSg48 were Propanoic acid, 2-hydroxy-, Benzene, 1,4-dimethyl-, Acetic acid, anhydride, Propanal, dimethylhydrazone, 1-Pentanol, 2,2-dimethyl-, Sulfurous acid, isohexyl 2-propyl ester, Oxalic acid, cyclohexyl dodecyl ester, 4-methyl oxazole, Oxalic acid, cyclohexyl tetradecyl ester, Oxalic acid, cyclohexyl pentyl ester, Acetic acid, sodium salt, and 1H-Indole-3-ethanamine, 6-fluoro -beta.-methyl-.
The results showed that the MVOC emitted by E. asburiae MjSg48 and contributed to increasing plant growth were
oxalic acid, cyclohexyl dodecyl ester and 4-methyl oxazole.The analysis result of MVOC compounds in E. asburiae TK24 using GC-MS showed that there were 27 compounds detected. Figure 5. shows the MVOC chromatogram of E. asburiae TK24.

Figure 5. The MVOC Chromatogram of E. asburiae TK24
The MVOC compounds detected in E. asburiae TK24 were Propanal, 2,3-dihydroxy-, Benzene, ethyl-, XYLENE, 4-Amino-furazan-3-carboxylic acid (2-acetylamino-ethyl)-amide, 1,2- Xylene, Butanoic acid, 2-ethyl-2-methyl-, Pentane, 3-ethyl-3-methyl-, Oxalic acid, isohexyl neopentyl ester, 2,4-Octanedione, Thiazole, 1-Pentanol, 2,2-dimethyl- , Oxalic acid, cyclohexyl decyl ester, Oxalic acid, cyclohexyl pentyl ester, Oxalic acid, cyclohexyl hexyl ester, N1-METHYL-1,4-BUTANEDIAMINE, 5-Hexen-2-one, 2,3-Anhydro-d-galactosan, 1,3-Propane diamine, N-methyl-, Benzene, 1,2,3,4-tetramethyl-, 4-
Heptanone, Heptanamine, 5-methyl-, Piperidine, 3,3-dimethyl-, Octadecane, Pentanal, Dibutyl phthalate, and 1,2-Benzenedicarboxylic acid, mono (2-ethyl) ester.
The results showed that the MVOC which emitted by E. asburiae TK24 and contributed to increasing plant growth were oxalic acid, isohexyl neopentyl ester, thiazole, oxalic acid, and cyclohexyl decyl ester. The results of MVOC compounds analysis in P. rettgeri Al2TT using GC-MS showed that there were 13 compounds detected. Figure 6. shows the chromatogram of MVOC in P. rettgeri Al2TT
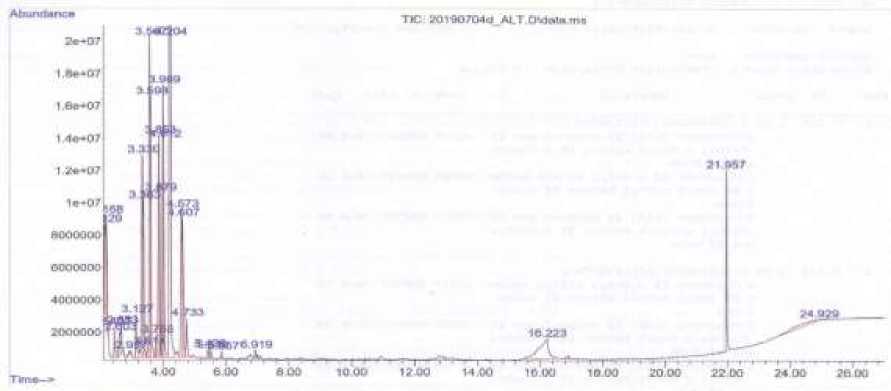
Figure 6. The Chromatogram of MVOC in P. rettgeri Al2TT
The MVOC compounds which detected in P. rettgeri Al2TT were Propanal, 2,3-dihydroxy-, Benzene, 1,4-dimethyl-,
Oxalic acid, diisohexyl ester, Octanal, 2,4-Pentanedione, Oxalic acid, cyclohexyl octyl ester, Oxalic acid, cyclohexyl tetradecyl ester, Pyridine, 2,3,4,5-tetrahydro-, Carbamic acid, (3,4,4-trimethyl-1, 2-dioxetan-3-yl) methyl ester, 1-Butanol, 2- amino-, delta. 2-tetrazaboroline, 5-ethyl-1,4-dimethyl-, 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, and 1,2-Benzenedicarboxylic acid. The results showed that the MVOC emitted by P. rettgeri Al2TT and contributed to increasing plant growth were oxalic acid, diisohexyl ester, Pyridine, 2,3,4,5-tetrahydro.
Discussions
A wide variety of microorganisms found in the rhizosphere are capable of
producing substances that regulate plant growth and development. Chemical signals from microbes have been found to play a role in plant morphogenetic processes, including N-acyl-L-homoserine lactones (AHL) and volatile organic compounds (VOCs). AHL is a bacterial quorum sensing signal from Gram-negative bacteria such as Pseudomonas. These compounds allow bacterial cells to regulate gene expression depending on population density. Recently, it was found that AHL can be recognized by plants, alter gene expression in roots and shoots also modulate cell growth defense and response. In the same way, bacterial volatiles such as acetoin and 2,3-butanediol produced by certain rhizobacteria can be used for bacterial and plant communication, as well as plant growth triggers. Acetoin VOC-producing bacteria were able to increase the
root growth of Lactuca sativa plants (Fincheira et al., 2016).
Paracoccus halophilus G062 emits 1,2-Dimetoxy-4-(2propenyl) benzene or Methyl eugenol (ME) compounds which can increase potato plant growth. B. subtilis GB03 which emits compounds 3-hydroxy-2-butanone, 2,3-butanediol, decanal, dean, tetramethyl pyrazine, and undecane which can increase the growth of A. thaliana (Ryu, 2004, Akhdiya, 2014, Ditengou, et al., 2015). The results of VOC trapping of B. subtilis G8 which was carried out using SPME fibers and followed by identification using GC-MS
CONCLUSION
Different bacterial species produce different MVOC. S. maltophilia Sg3 emitted 20 MVOC compounds and MVOC that contribute to increasing plant growth, namely oxalic acid, cyclohexyl undecyl ester, 2-Furancarboxaldehyde, 5-methyl-, 1,2 butanediol, and Piperazine. E. asburiae MjSg48 emitted 12 MVOC compounds and those that contributed to increasing plant
REFERENCES
Akhdiya, A., Wahyudi, A.T., Munif, A.,Darusman, L.K. 2014.
Characterization of an Endophytic Bacterium G062 Isolate with
obtained 30 types of compounds consisting of alkyl groups, alcohols, esters, ketones, acids, amines, oxime, phenol, and heterocyclic compounds. Ectomycorrhiza produce VOC sesquiterpene which able to increase the roots growth of A. thaliana. reported that Phoma sp. GS8-3 emits VOCs 2-Methyl-propanol, 3-Methyl-butanol, Phenyl ethyl alcohol, 3-Hydroxy-2-butanone, 2,3-Butanediold, 1-Octen-3-ole, Methacrylic acid, Isobutyl acetate, Acetic acid, Tiglic acid can increase the growth of tobacco plants (Liu et al., 2008, Naznin et al., 2013)
growth were oxalic acid, cyclohexyl dodecyl ester and 4-methyl oxazole. E. asburiae TK24 emitted 27 MVOC compounds and those that contributed to increasing plant growth were oxalic acid, isohexyl neopentyl ester, thiazole, Oxalic acid, and cyclohexyl decyl ester. Meanwhile P. rettgeri Al2TT emitted 13 MVOC compounds and those that contributed to increasing plant growth were oxalic acid, diisohexyl ester, and Pyridine, 2,3,4,5-tetrahydro.
Beneficial Traits. Hayati Journal of Biosiences. 21(4): 187-196.
Blom, D., Fabbri, C., Connor, E., Schiestl, F., Klauser, D., Boller, T., et al. (2011). Production of plant growth modulating volatiles is widespread among
rhizosphere bacteria and strongly depends on culture conditions. Environ. Microbiol. 13, 3047-3058.
Bohm, K., Martín-Sánchez, L.,& Garbeva, P. (2017). Microbial Volatiles: Small Molecules with an Important Role in Intra- and Inter-Kingdom Interactions. Frontiers in Microbiology, 8, 1-10.
doi:10.3389/fmicb.2017.02484.
Cappellari, L., & Chiappero, J., Palermo, T., Giordano, W. & Banchio, E. (2020). Volatile Organic Compounds from Rhizobacteria Increase the
Biosynthesis of Secondary Metabolites and Improve the Antioxidant Status in Mentha piperita L. Grown under Salt Stress. Agronomy, 10(8), 1-16. doi:
1094. 10.3390/agronomy10081094.
Castro, R.O., Hexon, A.C.C., Lourdes, M.R., Jose, L.B. 2009. The role of microbial signals in plant growth and development. Plant Signaling & Behavior 4(8): 701-712.
Ditengou, F.A., Anna, M., Maaria, R., Judith, F., Hanna, L., Maja, M., Valerie, L., Klaus, P. (2015). Volatile Signalling by Sesquiterpenes from Ectomycorrhizal Fungi Reprogrammes Root
Architecture. Nature Communications, 6, 1-9.
Ezquer, I., Li, J., Ovecka, M., Baroja-Fernandez, E., Jose Munoz, F., Montero, M., et al. (2010). Microbial volatile emissions promote
accumulation of exceptionally high levels of starch in leaves in mono- and dicotyledonous plants. Plant Cell Physiol, 51, 1674-1693.
Fincheira, P., Maribel, P., Andres, Q. (2017). Volatile Organic Compounds
Stimulate Plant Growing and Seed Germination of Lactuca Sativa.
Journal of Soil Science and Plant Nutrition, 17 (4), 853-86.
Gupta, A., Gupta, R., Singh, R.L. (2017). Microbes and Environment. In: Singh, R. (eds) Principles And Applications Of Environmental Biotechnology For A Sustainable Future. Applied Environmental Science and
Engineering for a Sustainable Future. Springer, Singapore.
https://doi.org/10.1007/978-981-10-1866-4_3.
Gustiar, F., Munandar, M., Amar, M., Arsi, A., Pitayati, P. A., Amanah, T. O., & Assyfa, N. (2022). Growth of Pakcoy (Brassica rapa L.) Hydroponic System Using Nutrients of Catfish Cultivation Waste. Jurnal Lahan Suboptimal: Journal of Suboptimal Lands, 11(1), 86–93.
https://doi.org/10.36706/jlso.11.1.2022 .560.
Kanchiswamy, C. N., Malnoy, M., & Maffei, M. E. (2015). Chemical Diversity of Microbial Volatiles and Their Potential for Plant Growth and Productivity. Frontiers in Plant Science, 6, 151. doi: doi.org/10.3389/fpls.2015.00151
Kai, M., Crespo, E., Cristescu, S. M., Harren, F. J., Francke, W., and Piechulla, B. (2010). Serratia Odorifera: Analysis of Volatile Emission and Biological Impact of Volatile Compounds on Arabidopsis Thaliana. Appl. Microbiol. Biotechnol. 88, 965-976.
Kanchiswamy, C.N. Mickael, M., Massimo, E.M. (2015). Chemical Diversity of Microbial Volatiles and Their Potential for Plant Growth and Productivity. Frontiers Plant Science, 6, 1-23.
Liu, W., Wei, M., Bingyu, Z., Feng, L. (2008). Antifungal Activities and Components of VOCs Produced by
Bacillus subtilis G8. Current Research in Bacteriology, 1(1), 28-34.
Orzechowski, S., Sitnicka, D., Grabowska, A., Compart, J., Fettke, J. & Zdunek-Zastocka, E. (2021). Effect
of Short-Term Cold Treatment on Carbohydrate Metabolism in Potato Leaves. Int. J. Mol. Sci, 22, 1-17.
doi://doi.org/10.3390/ijms22137203
Park, Y-S., Dutta, S., Ann, M., Raaijmakers, J. & Park, K. (2015). Promotion of Plant Growth by Pseudomonas Fluorescens Strain SS101 via Novel Volatile Organic Compounds. Biochemical and Biophysical Research Communications, 461(2), doi:
10.1016/j.bbrc.2015.04.039.
Ramirez, K., Lauber, L.L. & Fierer, N. (2010). Microbial Consumption and
Production of Volatile Organic Compounds at the Soil-Litter Interface. Biogeochemistry, 99(1), 97-107. doi: 10.1007/s10533-009-9393-x.
Ryu, C. M., Farag, M. A., Hu, C. H., Reddy, M. S., Wei, H. X., Pare, P. W., et al. (2003). Bacterial Volatiles Promote Growth in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A., 100, 4927-4932.
Ryu, C. M., Farag, M. A., Hu, C. H., Reddy, M. S., Kloepper, J. W., and Pare, P. W. (2004). Bacterial Volatiles Induce Systemic Resistance in Arabidopsis. Plant Physiol., 134, 1017-1026.
Schmidt, R., Cordovez, V., de Boer, W., Raaijmakers, J. & Garbeva, P. (2015). Volatile Affairs in Microbial Interactions. ISME J, 9, 2329–2335. doi://doi.org/10.1038/ismej.2015.42.
51
Discussion and feedback