THE EFFECTS OF DIETARY INCLUSION OF DETOXIFIED Leucaena leucocephala LEAF MEAL ON THYROIDAL ACTIVITY OF RATS DURING GESTATION-LACTATION PERIOD
on
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY • Vol. 5 No. 2 • April 2018
ISSN: 2303-3371
https://doi.org/10.24843/IJBB.2018.v05.i02.p02
THE EFFECTS OF DIETARY INCLUSION OF DETOXIFIED Leucaena leucocephala LEAF MEAL ON THYROIDAL ACTIVITY OF RATS DURING GESTATIONLACTATION PERIOD
Ngurah Intan Wiratmini1*, Inna Narayani1, and Ni Luh Eka Setiasih2
1Department of Biology, Faculty of Mathematics and Natural Sciences, Udayana University 2Faculty of Veterinary, Udayana University
*Coresponding Author: wiratminiintan@unud.ac.id
ABSTRACT
Leucaena leucocephala (L. leucocephala) provides the highest amount of proteins compared to other greens. However, its use is restricted due to the content of mimosine, an antinutrition non-protein amino acid known to be toxic to animals. This study aimed to observe the effect of administration of more than 10% of processed L. leucocephala leaf meal to the level of T3, T4 and the thyroid histopathology. In this study, L. leucocephala leaves were soaked in distilled water for 12 hours. The processed of L. leucocephala leaf meal was made into pellets containing 0%, 7.5%, 15%, and 22.5% leaf meal of total feeds respectively, and was fed to the rats during pregnancy and lactation. The level of triiodothyronine (T3) and thyroxine (T4), and the thyroid histological features were the parameters observed. The collected data were statistically analyzed by SPSS 20.0 for Windows using One-way ANOVA followed by Duncan Multiple Range Test to observe any significant difference among the 4 treatment groups (α=5%). Meanwhile, the presence of hyperplastic cells and follicle lumens filled with vesicles and colloids was descriptively analyzed by means of available literatures. The analysis of T3 and T4 level revealed that there was no any significant difference between the control and treatment groups. The epithelial cells of thyroid follicles in the treatment groups of P1, P2 and P3 showed hyperplasia and were detached from their respective basal membranes.
Keywords: leucaena, detoxification, thyroid, rats
INTRODUCTION
Leucaena leucocephala (L. leucocephala) leaves contain proteins as high as 25-35% of their dry weight (Gosh and Bandyopadhyay, 2007). In other hand, also contain mimosine, an antinutrition nonamino acid substance which has been known to cause several disorders in cattles, one of which is the inhibition of thyroid hormone synthesis due to its anti-thyroid activity (Komari, 1993). Additional 75% L.
leucocephala leaf meal in cow’s feed for more than 22 days caused a decline in serum level of T3 (triiodothyronine) and T4 (thyroxine) (Gosh et al., 2007). The decline of T3 and T4 blood serum level could cause thyroid gland enlargement, a condition called goiter. Ly et al. (2007) reported that excessive consumption of L. leucocephala by animals could lead to decrease in body weight, thyroid dysfunction, alopecia, and decline in both quality and quantity of
semen, thus impairing reproductive function. Mimosine exhibits negative effects not only in ruminants but also in monogastric animals, in which administration of more than 10% L. leucocephala leaf meal was conducted (Rai et al., 1992).
The mimosine content of L. leucocephala depends on the age of the plant, drying method (treatment), and season (Bray, 1994). Recently, researchers are focused to find a way to reduce the mimosine level contained in L. leucocephala leaf, thus reducing the exhibited hazard in animal wellbeing. The detoxified L. leucocephala leaves by means of water-soaking method reduced the mimosine content up to 73,31% (Wiratmini et al., 2014). The study also reported no abnormalities in the development of embryos while detoxified L. leucocephala leaf meal was incorporated into the feeds of the rats in pregnancy periode. Meanwhile, there is no research regarding the effect of detoxified leucaena by soaking in water to the subject of the thyroid gland. Therefore, it is of interest to study for the subject matter to the livestock that was given detoxified leucaena during pregnancy and lactation. In this study, albino rats (Rattus norvegicus) were used as models for monogastric cattle. The serum level of T3 (Triiodothyronine) and T4 (thyroxine) and thyroid histological features were used as indicators of thyroid gland activity.
MATERIALS AND METHODS
The Processing of Treatment Feed
L. leucocephala leaves were hand-picked from the trees that grow at the roadside of Bypass Ngurah Rai Street, Denpasar, Bali. The leaves were removed from its stem and let detoxified by soaking in cold water (pH 8.5) at 1 kg leaves-1 30 litre of water for 12 h. Treated leucaena were then collected by losing the water through sieve and immediately air-dried up until the constant dry weight is achieved. The leaves were later milled and sifted to produce L. leucocephala leaf meal according to Zakayo et al., (2000) which then made into pellets as such: The feeds used for the control group (P0) contained 0% of detoxified L. leucocephala leaf meal and consisted only of commercial pig feed CP 551, while the one used for treatment groups was a mix of CP 551 and detoxified L. leucocephala leaf meal designated as P1, P2, and P3. The mixing process was done by homogenization with the addition of 1.5% CMC and water. The mixture was then processed in pelleting machine and the resulting pellets were airdried in a 60oC oven. The finally processed pellets were stored in sealed clear plastic bags (Wiratmini et al., 2014).
Experimental Procedure
Female albino rats (Rattus norvegicus), approximately 3 months old and with an
average live weight of 180-200 g, were acclimatized inside a 33x25x14 cm plastic container for 7 days. The room where acclimatization took place was set in 25-26oC temperature with alternating 12 hours light and dark cycles. During this period, the rats were fed on CP 551 (produced by PT Charoen Pokphan Indonesia) with ad-libitum access to water. Estrous cycle was determined by vaginal smear and Giemsa staining method. Females in estrous were mated with males in the afternoon. A vaginal plug or sperm found on the next day indicated that copulation had occurred and this was taken as being the 0 day of gestation.
The Completely Randomized Design was used in this study, involving four treatment groups (P0, P1, P2 and P3) based on the level of incorporating L. leucocephala leaf meal in the given feeds, each group comprising eight rats. Thirty-two of albino rats in their 1st day of gestation were randomly grouped into the aforementioned treatment groups. The control group (P0) was fed only on CP 551 while P1, P2, and P3 were fed on treatment feed incorporated with 7.5%, 15%, and 22.5% of detoxified L. leucocephala leaf meal, respectively. The experiment was conducted for 42 days, counted from the 1st day of gestation until the end of lactating period. On the 43rd day, all rats were sacrificed by means of intramuscular ketamine injection and dissected to collect
the thyroid glands and blood samples. The blood samples were drawn from the orbital sinus using microhaematocrite tubes and collected in 1.5 ml microtubes. Collected blood samples were left at room temperature for 45 minutes, and then centrifuged for 10 minutes at 3000 RPM. The serum was transferred to other tubes and then evaluated for both the level of T3 and T4 measured by Vidas Test Kit from Biomerieux according to the steps instructed by the manufacturers. Dissected thyroid glands were made in histological preparations embedded in paraffin wax, stained by Haematoxylin-Eosin (HE), and observed by means of light microscope equipped with an Optilab camera in 400 x magnifications.
Data Analysis
SPSS 20.0 for Windows was used to statistically analyze the collected data. The T3 and T4 serum level were analyzed by One Way ANOVA for normally distributed data and followed by Duncan Multiple Range Test (DMRT) for significantly different ones. Thyroid gland histopathology was descriptively analyzed by referring to Kumar et al. (2007).
RESULTS AND DISCUSSION
The Serum Level of Triiodothyronine (T3) and Thyroxine (T4)
The mean serum level of T3 and T4 was presented on Table 1. The data suggested that
the serum level of T3 and T4 in the treatment groups tend to decline along the increased level of detoxified L. leucocephala leaf meal incorporated in the feeds, but they were not statistically different from the control group
(p>0.05). This implicates that incorporated detoxified L. leucocephala leaf meal up to 22.5% did not cause any decline on the serum level of T3 and T4.
Table 1. The mean serum level of triiodothyronine (T3) and thyroxine (T4) of gestating albino rats fed in feeding incorporated by detoxified Leucaena leucocephala leaf meal during gestation-lactation period.
|
Parameter Observed |
Treatment Groups | |||
|
P0 |
P1 |
P2 |
P3 | |
|
T3 (nmol/L) |
0,52±0,02a |
0,47±0,06a |
0,50±0,08a |
0,45±0,02a |
|
T4 (nmol/L) |
56,27±5,38a |
56,00±4,80a |
53,07±3,55a |
51,17±4,48a |
Note: values followed by the same superscripts in the same row were not statistically different (α=0.05). PO (control) = 100% feed pellet CP 551, P1= 7,5% leaf meal, P2=15% leaf meal, P3=22,5% leaf meal
The serum level of T3 and T4 did not decline significantly due to the fact that the detoxified L. leucocephala leaf meal used in this study contained less than 1% mimosine. Wiratmini et al. (2014) reported that the mimosine contain in 12-hours detoxified L. leucocephala leaves decreased up to 73.31%, which implicates that the mimosine level in the feeds of P3, that contained the highest amount of incorporated detoxified L. leucocephala leaf meal (22.5%), was only of 0.64%.
Monogastric livestock was reported to be intolerant of undetoxified L. leucocephala leaf meal if given more than 10% the total feed (Rai et al., 1992). According to Wiratmini (2014), undetoxified L. leucocephala leaf meal contained 10.64% mimosine, which means that feed incorporated with 10% L. leucocephala leaf
meal would contain 1.06% mimosine. Monogastric livestock, for example rabbits, were intolerant to even more than 1% of momosine as reported by Fayemi (2011). Chancay and Poosaran (2009) reported that monogastric livestock fed on more than 10% undetoxified L. leucocephala leaf meal eventually developed several disorders.
Gosh et al. (2007) reported that cows fed on undetoxified L. leucocephala leaf meal during lactation exhibited decreased of T3 and T4 serum level as well as goiter. Aside from cows, undetoxified L. leucocephala leaf meal also caused goiter in goats (Sastry et al., 2008). This phenomenon is due to the fact that mimosine is structurally similar to tyrosine, an amino acid that binds with iodide during the process of thyroid hormone synthesis (Hegarty et al., 1964). Therefore, mimosine acts as the competitive
inhibitor of tyrosine in terms of iodide binding, thus leading to the depletion of iodide ions as substrates for the synthesis of T3 and T4.
Wiratmini et al. (2014) reported that incorporation of up to 22.5% detoxified L. leucocephala leaf meal into the feeds of albino rats did not cause either abortions, postnatal death, developmental abnormalities, or decrease in the litter size. This implies that detoxified L. leucocephala leaf meal during the gestation period does not interfere with thyroid hormone synthesis in rats. Thyroid hormones serve as growth control, differentiation, as well as metabolism of all somatic cells (Grummer et
al., 1988). During gestation, thyroid hormones function in the maturation and providing nutrition for the uterus, including placental growth, fetus metabolism, and production of other hormones related to growth (Glinoer, 1997). Inadequate level of thyroid hormones could lead to infertility and impaired reproductive functions (Airin, 2011).
Histological Features of Thyroid Gland
The histological features of thyroid glands in albino rats fed with feeding incorporated by detoxified L. leucocephala leaf meal was shown in Table 2.
Table 2. Histological features of thyroid glands in albino rats fed with feeding incorporated by detoxified Leucaena leucocephala leaf meal during gestation-lactation period.
|
Parameters Observed |
Treatment Groups | |||
|
P0 |
P1 |
P2 |
P3 | |
|
Hyperplasia |
- |
+ |
+ |
+ |
|
Colloids |
+ |
- |
- |
- |
|
Hemorrhage |
- |
- |
- |
- |
|
Shape of follicles |
Organized |
Disorganized |
Disorganized |
Disorganized |
|
Shape of epithelial cells |
Cuboidal |
Cuboidal |
Cuboidal |
Cuboidal |
|
Position of epithelial cells |
Attached to basal membrane |
Detached |
Detached |
Detached |
Note : - not found
+ found
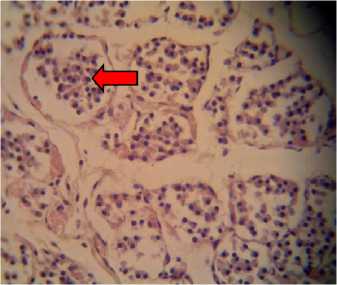
The histological features of thyroid glands found in the control group were of organized follicles filled with pinkish colloids, arranged by cuboidal epithelial cells that were attached to their respective basal membrane. A colloid is a mixture of proteins
consisting of thyroglobulins which would be broken down to T3 and T4 by endopeptidase enzyme. The resulting hormones would be released into the bloodstream before reaching their target organs (Ganong et al., 2008). Meanwhile, the histological features found in
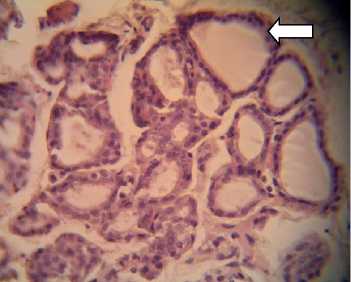
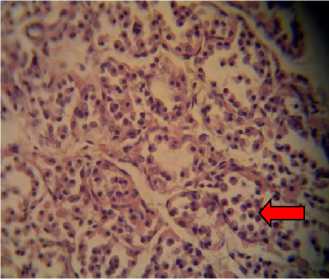
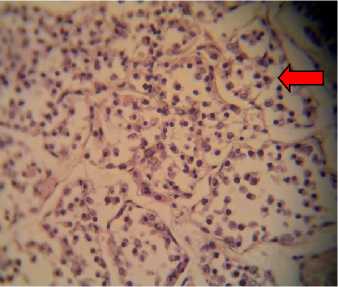
P1, P2, and P3 were of hyperplastic cells that were detached from their respective basal membrane, which lead to the disorganized appearance of the follicles that contained neither colloids nor hemorrhage (Fig. 1). Those mentioned findings in P1, P2, and P3
suggested that mimosine contained in detoxificated L. leucocephala leaf meal was responsible for producing such histological features. Hyperplasia of follicle cells causes enlargement of the thyroid gland, a condition known as goiter.
A
B
C
D
Fig. 1. Histological preparations of thyroid glands in albino rats fed with feeding incorporated with detoxified Leucaena leucocephala leaf meal, viewed under a light microscope. A: P0, in which thyroid follicles consisted of cuboidal epithelial cells (white arrow); B: P1; C: P2; and D: P3, in which epithelial cells were hyperplastic and detached from their respective basal membrane (red arrow).Viewed in 400x magnifications.
Hyperplasia is the main response of tissues due to physiological and pathological stress to achieve a new equilibrium state and ensure survival (Kumar et al., 2007). In this condition, the cells are said to be undergoing adaptation process. If the insulting factors were to be removed before further cellular
damage took place, those cells would return to their initial state.
CONCLUSIONS
The incorporation of detoxified L. leucocephala leaf meal to the feeds of albino rats during gestation-lactation period did not
cause any decline in the serum level of T3 and T4 while the histological features of thyroid glands suggested hyperplasia of epithelial cells that were detached from their respective basal membrane.
ACKNOWLEDGEMENTS
The author would like to thank Lembaga Penelitian dan Lembaga Penelitian dan Pengabdian Kepada Masyarakat Universitas Udayana and Direktorat Penelitian dan Pengabdian Kepada Masyarakat Dirjen Dikti who has provided Grant Funding Research, with the Letter of Agreement of Research Implementation Number 311-
133/UN14.2/PNL.01.03.00/2015, March 3, 2015.
REFERENCES
Airin C., Prabowo, P., Pudji, A., Endang, B., Sunaryanto, & Didik, Y. (2011). Level Hormon Triiodothyronine dan
Thyroksin Saat Estrus dan Ovulasi Pada Sapi Bali. J Sain vet, 29(1), 3742.
Bray, R. (1994).The Leucaena Psyllid. In: Gutteridge RShelton H, ed. by. Forage tree legumes in Tropical Agriculture [Internet]. 1st ed. United Kingdom: CAB International; [cited 2 October 2012].
Chanchay, N. & Poosaran, N. (2009). The reduction of mimosine and tannin contents in leaves of Leucaena leucocephala. Asian Journal of Food and Agro-Industry. Special Issue. 137144.
Fayemi, P. O., Onwuka, C. F. I., Isah, O. A., Jegede, A. V., Arigbede, O. M., & Muchenje, V. (2011). Effects of
mimosine and tannin toxicity on rabbits
fed processed Leucaena leucocephala (Lam) De Wit. Leaves. African J. Of Agri. Research, 6 (17), 4081-4085.
Ganong, W. (2001). Review of Medical Physiology. 20th ed. Appleton & Lange.
Ghosh, M.K., Atreja, P.P., Buragohain, R. and Bandyopadhyay, S. (2007).
Influence of short-term Leucaena leucocephala feeding on milk yield and its composition, thyroid hormones, enzyme activity, and secretion of mimosine and it metabolites in milk of catlle. The Journal of Agricultural Science, 145(4), 407- 414.
Ghosh, M.K., and Bandyopadhyay, S. (2007). Mimosine toxicity-a problem of leucaena feeding in rumaninants. Asian Journal of Anim. and Vet. Advences, 2 (2): 63-73.
Glinoer, D. (1997). The Regulation of Thyroid Function in Pregnancy: Pathways of Endocrine Adaptation from Physiology to Pathology. Endocrine Reviews, 18(3):404-433.
Grummer R, Carroll D. (1988). A Review of Lipoprotein Cholesterol Metabolism: Importance to Ovarian Function. Journal of Animal Science, 66(12),
3160.
Hegarty, M., Schinckel, P., & Court, R.
(1994). Reaction of sheep to the consumption of Leucaena glauca
Benth. and to its toxic principle mimosine. Aust J Agric Res, 15(1),
153.
Komari. (1993). Composition of Leucaena Tempe. ASEAN Food Journal, 8(4), 157-158.
Kumar, V., Abbas, A. K., Fausto, N., & Mitchell, R. (2007). Basic Pathology. 8th ed. Philadelphia: W. B. Saunders.
Ly, J., Grageola, F., Lemus, C., Castro, M. (2007). Iileal and Rectal Digestibility of Nutrients in diets based on Leucaena leucocephala (Lam) de Wit for pigs. Influence of the inclusion of zeolite. Journal of Anim Vet Adv, 6(12), 13711376.
Rai, A., Khanna, N., & Argawal, S. (1992).
Effect of feeding Leucaena
leucocephala with Phaseolus
aconitifolius on growth and thyroid status of camel calves. J Anim Sci, 62, 297-301.
Sastry, M. S., Singh, & Rajendra. (2008).
Toxic effects of subabul Leucaena leucocephala on the thyroid and reproduction of female goats. Indian Journal of Animal science, 78(3):251-253.
Wiratmini, N. I. (2014). Penampilan Reproduksi, Proliferasi dan Aktivitas Sel Sekretori Kelenjar Mammae Tikus Putih yang Diberi Daun Lamtoro (Leucaena leucocephala) Hasil
Perendaman (dissertation). Denpasar: Universitas Udayana.
Wiratmini, N., Oka, I., & Mahardika, I.
(2014). The Effect of Detoxificated
Leucaena leucocephala Leaf Meal to
Prenatal Development of Wistar Rat
Fetus. Int Journal Pure App Biosci, 2(6):223-227.
Zakayo, G., Krebs, G .L., & Mullan, B. P.
(2000). The use of Leucaena leucocephala leaf meal as protein supplement for pigs. Asian-Aus. J. Anim. Sci, 13(9), 1309-1315.
ASIA OCEANIA BIOSCIENCE AND BIOTECHNOLOGY • 110
Discussion and feedback