GENETIC DIVERSITY AND FRUIT QUALITY OF SEVERAL POMELO “JERUK BALI” (Citrus grandis L. Osbeck) CULTIVARS IN BALI
on
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY • Vol. 5 No. 1 • September 2017
ISSN: 2303-3371
https://doi.org/10.24843/IJBB.2017.v05.i01.p04
GENETIC DIVERSITY AND FRUIT QUALITY OF SEVERAL POMELO “JERUK BALI” (Citrus grandis L. Osbeck) CULTIVARS IN
BALI
Ida Bagus Komang Mahardika1*, I Nyoman Rai2, Made Sudiana Mahendra2, and 2
Rindang Dwiyani
1Department of Agrotechnology, Faculty of Agriculture Warmadewa University 2 Department of Agroecotechnology, Faculty of Agriculture
Udayana University
-
*Corresponding author: gusmahardika62@gmail.com
ABSTRACT
This study aimed to determine the genetic diversity and fruits quality of the "Jeruk Bali" cultivars grown in Bali. This research was conducted in all regencies and city in Bali, during 2016. Furthermore, several cultivars of “Jeruk Bali” were genetically analyzed based on RAPD markers using 10 primers. Analysis of the quality of fruit is based on physical properties and chemical content. Eighteen cultivars of "Jeruk Bali" obtained have a fruit morphological character with round, short round, and piriform fruit shapes, which are red, pink, cream and white flesh color. RAPD analysis results at 53% similarity level are grouped into 5 groups. The first group was only one cultivar, the second group consisted of 13 cultivars, the third and fourth groups were only one cultivar, while the fifth group consisted of two cultivars. The analysis of the diversity between cultivars based on the combination of physical and chemical properties of the fruit with hierarchy method on similarity level about 85% in a group is obtained by 4 (four) groups. Groupings by combination of physical and chemical properties of the fruit are not synchronized in their entirety with dendograms based on their genetic diversity. This illustrates the physicochemical properties of “Jeruk Bali” fruit in general is not fully genetical expressed, but also influenced by conditions of environmental growth.
Keywords: “Jeruk Bali”, genetic diversity, fruit quality
INTRODUCTION
"Jeruk Bali" or pummelo (shaddock) (Citrus grandis L. Osbeck) is native to Indonesia, widely cultivated in India, China, Thailand and other East Asian
regions (Davies and Albrigo, 1994), needs to be handled seriously in cultivation and utilization in Indonesia (Setiawan and Sunarjono, 2003). “Jeruk Bali” has another name C. maxima (Burm.) Merr., C. decumana L., C. aurantium var. Grandis L.
and C. aurantium var. Decumana L. (Niyomdham, 1992; Setiawan, 1999; Hamilton, et al. 2008). “Jeruk Bali” is a good source of vitamin C and antioxidants (Rahman et al., 2003; Pichaiyongvongdee and Haruenkit, 2009).
Identification of genetic diversity of crop cultivars needs to be done for use in their development and cultivation. In addition, the evaluation of fruit quality also needs to be done to identify the nutritional compound of fruit as well as to know the response of plant varieties to the changing of the growing environment (Somantri et al., 2008). Biochemical analysis with isoenzymes in citrus plants, among others, is also used to distinguish the acidity level of citrus cultivars (Rahman et al., 2001). Excellence pomelo cultivars are generally determined based on the delicious fruit flavors (sweet and sour taste balance) as well as the water content of the juice (juicy)
(Rahman et al., 2003; Paudyal and Haq 2007).
"Jeruk Bali" fruit are generally considered to be ripe when the fruit skin color, the content of the juice and the ratio of total soluble solids with acidity and other internal components have reached the level of acceptance of visual minimum or palatability of consumers (Paudyal and Haq, 2007; Rukmana, 2009; Lado, et al., 2014; Riaz, M. et al, 2015 ). "Jeruk Bali" fruits are a source of calories, minerals and excellent for relieving thirst due to high water content (up to 86 g per 100 g of fruit flesh). The leaves can be used for epilepsy, chorea, and cough seizures (Niyomdham, 1992; Direktorat Tanaman Buah, 2012).
RAPD molecular markers are PCR applications used to detect the presence of a DNA polymorphism in a population or interpopulation. Polymorphisms produced by PCR RAPD techniques are due to changes in nucleotide bases, deletions, and
insertions (William et al., 1990). The advantage of the RAPD PCR technique is that it takes only a small quantity of DNA samples, is cost effective, easy to learn, and easy to obtain primers (Azrai, 2005). The use of molecular markers with RAPD PCR techniques has been widely used for early plant breeding activities, such as analyzing the genetic diversity of wani Bali (Rai, I N., et al., 2008), identifying genetic diversity among barley cultivars (Fernández et al., 2002), and detecting polymorphisms in distance plants due to gamma-ray radiation (Dhakshanamoorthy, D. et al., 2010). Evaluation of fruit quality including total soluble solids content (TSS), total titrated acids (TA), vitamin C, and flavonoid compounds (Pichaiyongvongdee and Haurenkit, 2009), are required to supplement the description of the "Jeruk Bali" that were grown in Bali.
MATERIALS AND METHODS
This research was conducted in all regencies/cities in Bali province. Molecular analysis was conducted in the laboratory of Genetics and Plant Breeding of Faculty of Agriculture UGM, and fruit quality observation was done at Agricultural Technology Technology Laboratory of Faculty of Agricultural Technology, Udayana University, Denpasar.
The method of genetic diversity testing using Random Amplified Polymorphic DNA (RAPD) method, while observation of fruit quality evaluation is done by sensory analysis (organoleptic) and analysis of chemical compound (nutrient) content according to the method of observation of each specification of the compound. DNA extraction methods (Doyle and Doyle, 1990) use the materials used are fresh "Jeruk Bali" leaves. The purified DNA is then quantified by using
Gene Quant to determine the concentration of DNA obtained.
DNA amplification is done by PCR (Polymerase Chain Reaction) reaction which aims to multiply DNA sequences based on the primary used. The PCR reaction was performed at a total volume of
-
10 μl for each PCR tube. Each PCR reaction consisted of 5 μl Go Taq® Green (Promega) PCR mixtures, 0.25 μl 100 μM primers (Sigma-Proligo), 2.5 μl sample DNA (template) and 2.25 sterile aquabidest. DNA amplification is done with PCR System BOECO tool. The DNA of PCR was then electrophoresed using 1.0% (w/v) agarose which has been added florosafe DNA stain as dye, in TBE buffer with 100 volt for 45 min. The results were then visualized with UV light. The DNA data obtained was analyzed using binary data by scoring, ie score 1 for the emerging band and score 0 for the band that did not appear (Williams, JGK., et al., 1990). The data
obtained by scoring the electrophoresis results for each individual on a certain size, and the binary data is then analyzed using the help of software Genalex 6.1 and Ntsys pc 2.2.
Observation of fruit quality evaluation used three pieces of ripe and healthy fruit in accordance with the criteria and characteristics, namely: smooth shiny smooth fruit skin, the colors are brighter and feels heavy, with PTT content around 7 - 10 oBrix, generally between 24 - 30 weeks after flowering (Setiawan, 1999; Mahardika and Susanto, 2003; Lado, J. et al., 2014). Sensory analysis in this study was conducted to identify consumer preferences and match the acceptability level of citrus fruits from various cultivars. The variables observed in fruit quality evaluation are: (a) percentage of fruit parts, including edible portion; (b) the vitamin C content of the fruit was measured using iodometric titration method (Cahyadi, 2006); (c) fruit
juice pH measured with Jenway 3010 digital pH meter; (d) total titrated acid content (TA) was measured by titration using 0.1 N NaOH until the pH of fruit juice reached 7 (AOAC, 2005); (e) total soluble solids (TSS) content, calculated as
the degree of Brix (oBrix) measured using a refractometer; (f) flavonoid levels were performed by spectrophotometry using aluminum chloride reagents (Chang and Wen, 2002); (g) sensory analysis performed ie. hedonic test and scalar quality test. The test was conducted by 10 semi-trained panelists, consisting of students and
laboratory technicians of the Faculty of Agricultural Technology Udayana University (Wagiyono, 2003).
RESULTS AND DISCUSSIONS
The genetic diversity of "Jeruk Bali " cultivars in Bali is based on RAPD marker test. Based on the varied distribution and morphological features, and the origin of the "Jeruk Bali " plant, 18 cultivars were selected to test their genetic diversity with RAPD markers. The list of samples analyzed using codes in accordance with the district / municipal sites where the plants were grown (Table 1).
Table 1. List of samples "Jeruk Bali" which analyzed
|
No. |
Local name |
Sample Code |
|
1. |
Juuk Bone Badung |
BAD-01 |
|
2. |
Juuk Saba Badung |
BAD-02 |
|
3. |
Jerungga Bangli |
BAN-03 |
|
4. |
Juuk Saba Badung |
BAD-03 |
|
5. |
Juuk Bali Jembrana |
JEM-02 |
|
6. |
Muntis Buleleng |
BUL-01 |
|
7. |
Juuk Bali Jembrana |
JEM-01 |
|
8. |
Juuk Bali Jembrana |
JEM-03 |
|
9. |
Muntis Buleleng |
BUL-02 |
|
10. |
Muntis Buleleng |
BUL-03 |
|
11. |
Jeruti Karangasem |
KAR-01 |
|
12. |
Juuk Saba Denpasar |
DEN-02 |
|
13. |
Juuk Saba Denpasar |
DEN-03 |
|
14. |
Juuk Saba Denpasar |
GIA-02 |
|
15. |
Jeruti Karangasem |
KAR-02 |
|
16. |
Juuk Bone Gianyar |
GIA-01 |
|
17. |
Jerungga Klungkung |
KLU-01 |
|
18. |
Juuk Bali Tabanan |
TAB-01 |
Stages of RAPD analysis is started from DNA extraction, DNA quantification, DNA dilution, DNA amplification and electrophoresis. DNA amplification is done by PCR reaction. Ten selected primers were applied the DNA obtained from the extraction of the "Jeruk Bali" samples of Bali, in the order of nucleotide bases as shown in Table 2. DNA concentrations in this study were obtained from 400 ng / μl in the 'Juuk Saba' Denpasar (DEN-03) and 'Jerungga' Klungkung (KLU-01) cultivars, up to 3996 ng / μl in the 'Juuk Saba' Badung (BAD- 02), while the purity of DNA obtained from 1.33 to 2.00. DNA purity is determined by the ratio of A260 / A280 (Sambrook et al., 1989; Chen, H. et al., 2010). The number of amplified DNA
fragments obtained in this study ranged from 5 to 15 bands depending on the type of primer and the genotype being analyzed. The smallest amount of DNA fragment (5) was obtained on the primary use of OPA-8, while the highest number of DNA fragments (15) was obtained on primary use of OPD-8 (Table 2). The ribbon pattern on agarose gel which is the result of genomic DNA amplification can be grouped into two categories: polymorphic band and monomorphic band. Polymorphic bands are a picture of DNA bands that appear at certain sizes, but in other samples no DNA bands are found in these sizes. The monomorphic band is a band in some samples that has no variation (Williams et al., 1990).
Table 2. Primers and their sequences, and the number of DNA bands umplified from 18 cultivars of jeruk bali pomelo in Bali
Primer Sequences Number of bands Total
|
5’ – 3’ |
Monomorphic |
Polymorphic | ||
|
OPA-1 |
CAGGCCCTTC |
0 |
13 |
13 |
|
OPA-2 |
TGCCGAGCTG |
0 |
9 |
9 |
|
OPA-5 |
AGGGGTCTTG |
0 |
7 |
7 |
|
OPA-7 |
GAAACGGGTG |
0 |
9 |
9 |
|
OPA-8 |
GTGACGTAGG |
0 |
5 |
5 |
|
OPD-2 |
GGACCCAACC |
2 |
10 |
12 |
|
OPD-3 |
GTCGCCGTCA |
0 |
10 |
10 |
|
OPD-5 |
TGAGCGGACA |
0 |
9 |
9 |
|
OPD-7 |
TTGGCACGGG |
0 |
12 |
12 |
|
OPD-8 |
GTGTGCCCCA |
1 |
14 |
15 |
|
Total |
3 |
98 |
101 | |
|
Persentase |
(%) |
2,97 |
97,03 | |
The result of visualization of DNA amplification with 10 genetic diversity genotypes 18 (eighteen) Bali citrus cultivars based on RAPD marker test is presented in Fig. 1. Dendogram profile of genetic diversity is presented in Fig. 2. The result of genetic diversity analysis of 18 Balinese citrus cultivars based on RAPD marker test with 10 primers at similarity level about 53 percent yielded 5 (five) groups, that is first group (I) consisted of one cultivar. Second group (II) consists of 13 cultivars, third
group (III) and fourth group (IV) each only one cultivar and the fifth group (V) consists of two cultivars. It appears that at a similarity level of 53 percent, some cultivars are specifically separated ie Gianyar 'Juil Bone' cultivars (GIA-01) as well as 'Jerungga' Klungkung which is a group with 'Juuk Bali' Tabanan (TAB-01). The separation of Gianyar 'Juice Bone' cultivars (GIA-01) corresponds to some of the specific properties possessed by these cultivars that are different from those of other cultivars.
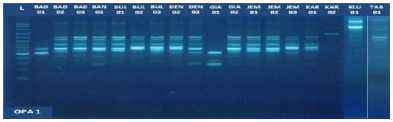

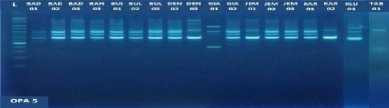

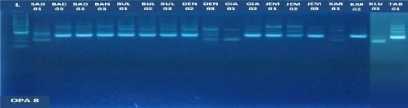
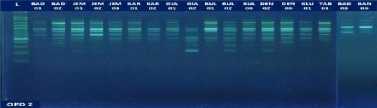
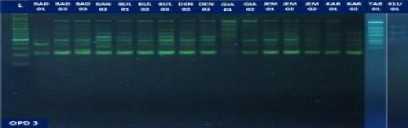
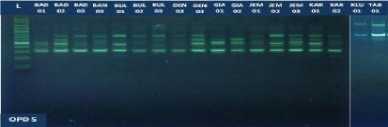
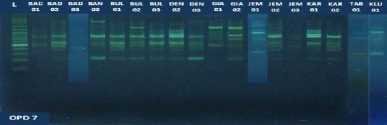
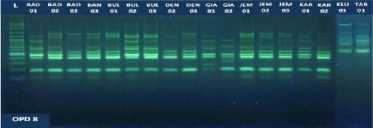
Fig. 1. DNA amplification with 10 primers of 18 (eighteen) pomelo cultivars based on
RAPD method
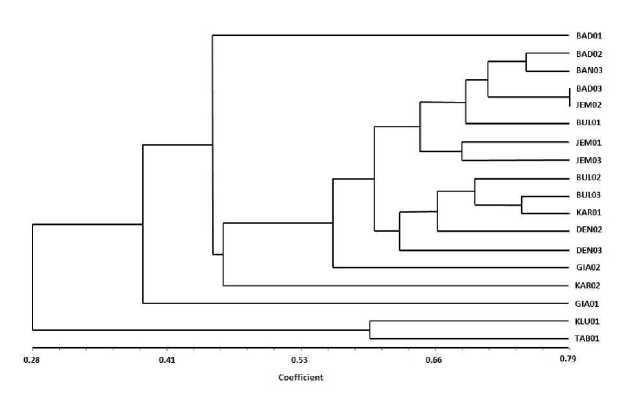
Fig. 2. Dendogram profile of genetic diversity of 18 (eighteen) “Jeruk Bali” cultivars based on RAPD marker test
DNA profile of 18 cultivars of pomelo in Bali which was amplified by using primer OPA-1, OPA-5, OPA-7, 3- OPD, OPD-7 and OPD-8 showed a specific band that characterizes some distinguishing trait for kultivar- Certain cultivars with other cultivars. For example, the primary use of OPA-1 there are specific band OPA-1_ 350 shows as a differentiator pomelo 'Juuk Bone' Gianyar (GIA-01) with other pomelo cultivars (Fig. 3).
Physical characteristics include fruit weight, skin color and fruit flesh (Fig. 4), portions of edible parts per fruit and several other physical characteristics. While the chemical character of the fruit is intended to contain some chemical compounds or nutrients present in the flesh of grapefruit, such as vitamin C content, total acids, total dissolved solids, and flavonoids in fruit
juice. The color of “Jeruk Bali” bark on observed cultivars ranges from bright green, yellowish green to yellow. While the color of juice sac varied, ranging from whitepink, reddish to red white (Table 3; Fig. 4.). Red-colored juice generally contains higher antioxidants than brightly colored juices (Pichaiyongvongdee and Haurenkit, 2009). So the possibility of 'Jeruti' Karangasem and 'Juuk Saba' Denpasar has higher antioxidant content than other cultivars. The weight of the average fruit portions are presented in Table 4.
The average pH of the fruit juice fruits ranged from 3.29 on 'Jembran Bali' Jembrana (JEM-03) to 4.89 in 'Bone Badung' (BAD-01) as indicated in Table 5. That were not much different from the pH of grapefruit juice in general and also reported in India that it ranges from 2.94 to 4.44 (Kumar, D. et al., 2015).
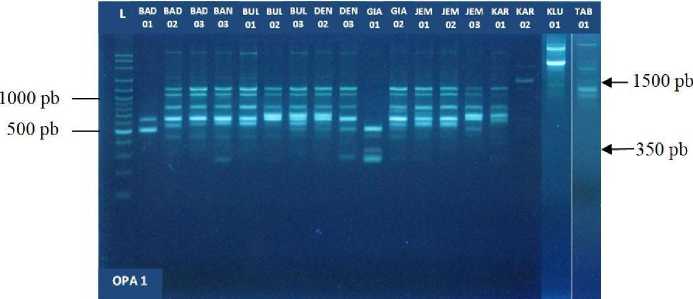
Fig. 3. Profile DNA 18 “Jeruk Bali” cultivars amplification product by using primer OPA-1. The specific band OPA-1_ 350 is the distinction of 'Juuk Bone Gianyar' (GIA-01) (column 10) with other cultivars. And the specific band OPA-1_ 1500 is the differentiator of the 'Jeruti' Karangasem (KAR-02) cultivar (column 16) with other cultivars. L = 1 Kb DNA Ladder
The total titrated acids content (ATT) of Bali oranges in Bali ranges from 0.44% -0.96 % (Table 5.5). The total titrated acids content is almost the same as that reported for pummelo in India which also ranges from 0.50 - 4.00 percent (Singh, S.K. et al., 2015). The total dissolved solids content (PTT) of “Jeruk Bali” juice in Bali is obtained from 7.00 oBrix in 'Jerungga' Bangli (BAN-03) to the highest of 11.77 oBrix at 'Juja Saba' Badung (BAD-03). This total dissolved solids content is almost the same as that reported for grapefruit
(pummelo) in India which also ranges from 7.40-11.50 oBrix. (Singh, S.K., et al., 2015). Sugar in oranges consists mostly of glucose, fructose and sucrose, and has increased during the fruit ripening period (Mahardika and Susanto, 2003).
Total flavonoid levels obtained in grapefruit juice in Bali ranged from 0.61 to 4.99 mg / 100 g (Table 5). Flavonoids are known to be antioxidant compounds that have a very important role in health, for example suspected to have cardioprotective effects and antiproliferative activity (Redha,
A., 2013). The highest total relative flavonoid levels in this study were obtained at Bangli (Jerk) cultivars (BAN-03) which reached 4.99 mg / 100g, much higher than those of other cultivars. This makes the Bang Bang fruit taste "Jerungga" has a harder taste ('pengah') that is harder than the others, because of the nature of flavonoid compounds that can cause bitter taste or bitter on the fruit. The research report Febrianti and Sari (2016) suggested that total flavonoid levels in some tropical fruits such as avocado, guava, citrus fruit, strawberry fruit, papaya fruit, tamarind fruit, apples and mangoes. While in research conducted by Selawa et al. (2013), about the flavonoid content of dry and wet leaf binahong leaves, concluded that dry binahong leaf extract is higher in flavonoid levels.
"Jeruk Bali" cultivars observed in this study in general were that have been
categorized excellent based on consumer opinions, and the communities around these crops are cultivated. The distinctive characteristic of this sensory judgment besides taste, sweet, and sour also has a distinct bitter bitter taste in Bali called the "pengah" which is a combination of bitter taste and an aromatic gas flavor in the nose. The preferred sensory character of the consumer is owned by almost all observed cultivars, which get a value greater than 4 (four) (Table 6). Three cultivars that have the unpleasant taste were 'Jeruti' Karangasem (KAR-02), 'Juuk Bali' Jembrana (JEM-03), and 'Jerungga' Bangli (BAN-03) have similarities from the height of the growing sites, about 500 meters above sea level.
The analysis of the diversity between cultivars based on a combination of physical and chemical properties of the fruit by hierarchial cluster analysis (Johnson & Wichern., 2007), at 85%
similarity level in a cluster Also formed 4 (four) clusters (Fig. 5.11). As with grouping according to physico-nature and chemical
properties, the combination is also not fully synchronized with dendograms based on its genetic characteristics.

Fig. 4. Shape and colour of fruit “Jeruk Bali” cultivars in Bali
This illustrates the physico-chemical properties of grapefruit in Bali in general does not show any major differences, except when viewed based on the content of certain compounds, the color of the flesh,
the total flavonoid content, the acid content, and the vitamin C, or the combination of these components and also the growing environmental influences.
The combination of certain properties between physics with clams and morphology is commonly used for the cultivar of the community. For example by mentioning the description of each cultivar found. However, for the purposes of certification of cultivar (fruit) products in
order to meet the needs of modern markets, the mention of a cultivar description should also be based on the grouping of its genetic diversity in addition to the mention of morphological identification, the chemical physics of the fruit and the location of its growing cultivation.
Table 5. Content of chemical compound of 18 “Jeruk Bali” fruit cultivars in Bali
|
Cultivar |
Vit. C content |
TSS content |
Total titrated |
pH |
Total Flav. |
|
Code |
(mg/1 00g) |
(oBrix) |
acids (%) |
(mg/100 g) | |
|
BAD-01 |
68,95 + 1,01 |
10,00 + 0,00 |
0,44 + 0,02 |
4,89 + 0,01 |
0,61 + 0,01 |
|
BAD-02 |
61,23 + 1,75 |
8,00 + 0,00 |
0,58 + 0,17 |
4,23 + 0,03 |
0,65 + 0,01 |
|
BAN-03 |
36,70 + 2,31 |
7,00 + 0,00 |
0,59 + 0,23 |
4,45 + 0,04 |
4,99 + 0,02 |
|
BAD-03 |
40,76 + 0,50 |
11,77 + 0,06 |
0,68 + 0,04 |
3,80 + 0,00 |
3,52 + 0,01 |
|
JEM-02 |
76,32 + 1,33 |
10,00 + 0,00 |
0,57 + 0,13 |
3,62 + 0,02 |
0,61 + 0,01 |
|
BUL-01 |
37,12 + 0,51 |
9,10 + 0,10 |
0,56 + 0,01 |
4,33 + 0,06 |
2,31 + 0,02 |
|
JEM-01 |
24,71 + 0,50 |
8,00 + 0,00 |
0,56 + 0,12 |
3,54 + 0,05 |
0,61 + 0,01 |
|
JEM-03 |
44,87 + 4,08 |
8,91 + 0,01 |
0,78 + 0,01 |
3,29 + 0,02 |
2,14 + 0,01 |
|
BUL-02 |
45,36 + 0,50 |
11,00 + 0,00 |
0,79 + 0,01 |
3,87 + 0,06 |
3,78 + 0,02 |
|
BUL-03 |
50,88 + 2,52 |
10,00 + 0,00 |
0,76 + 0,01 |
3,99 + 0,01 |
2,14 + 0,00 |
|
KAR-01 |
36,34 + 2,76 |
8,50 + 0,00 |
0,36 + 0,02 |
4,62 + 0,01 |
1,31 + 0,14 |
|
DEN-02 |
50,88 + 2,52 |
10,00 + 0,00 |
0,61 + 0,01 |
4,39 + 0,02 |
1,94 + 0,02 |
|
DEN-03 |
34,18 + 0,00 |
10,00 + 0,00 |
0,65 + 0,10 |
4,01 + 0,01 |
2,26 + 0,02 |
|
GIA-02 |
34,99 + 0,24 |
11,00 + 0,00 |
0,96 + 0,01 |
3,70 + 0,00 |
3,87 + 0,01 |
|
KAR-02 |
41,96 + 0,46 |
10,00 + 0,00 |
0,88 + 0,01 |
3,98 + 0,01 |
3,53 + 0,01 |
|
GIA-01 |
57,23 + 1,31 |
9,07 + 0,06 |
0,54 + 0,13 |
3,80 + 0,03 |
0,61 + 0,01 |
|
KLU-01 |
44,23 + 1,01 |
9,00 + 0,00 |
0,71 + 0,07 |
4,44 + 0,01 |
3,32 + 2,44 |
|
TAB-01 |
52,34 + 1,00 |
9,00 + 0,00 |
0,46 + 0,05 |
4,32 + 0,01 |
1,99 + 0,03 |
Table 6. Value hedonic test panelists on juice fruit 18 cultivars of fruit pomelo in Bali
|
Cultivar Code |
Value hedonic test *) | ||||
|
aroma |
taste |
colour |
juicines |
Average | |
|
BUL-03 |
4,10 |
4,85 |
4,90 |
4,55 |
4,60 |
|
KAR-01 |
4,20 |
4,90 |
4,70 |
4,40 |
4,55 |
|
DEN-03 |
4,30 |
4,80 |
4,70 |
4,30 |
4,53 |
|
GIA-01 |
4,35 |
4,70 |
4,35 |
4,35 |
4,44 |
|
TAB-01 |
4,45 |
4,30 |
4,55 |
4,00 |
4,33 |
|
JEM-01 |
4,19 |
4,38 |
4,43 |
4,14 |
4,29 |
|
JEM-02 |
4,05 |
4,05 |
4,25 |
4,10 |
4,11 |
|
DEN-02 |
4,10 |
4,20 |
3,70 |
3,90 |
3,98 |
|
BAD-02 |
3,75 |
4,10 |
4,30 |
3,75 |
3,98 |
|
BUL-01 |
3,55 |
4,15 |
4,15 |
4,00 |
3,96 |
|
BAD-01 |
4,10 |
4,40 |
3,15 |
4,15 |
3,95 |
|
KLU-01 |
3,15 |
4,30 |
4,05 |
4,15 |
3,91 |
|
BUL-02 |
3,90 |
4,25 |
3,60 |
3,70 |
3,86 |
|
BAD-03 |
3,45 |
3,40 |
3,85 |
4,15 |
3,71 |
|
GIA-02 |
3,55 |
4,20 |
3,75 |
3,00 |
3,63 |
|
BAN-03 |
4,25 |
2,50 |
3,00 |
3,35 |
3,28 |
|
KAR-02 |
2,90 |
3,30 |
3,60 |
2,00 |
2,95 |
|
JEM-03 |
3,45 |
3,15 |
3,10 |
2,00 |
2,93 |
Description: *) 1 = dislikes; 2 = rather dislike; 3 = neutral; 4 = rather like; 5 = likes
Clustering Jeruk Bali Menurut Sifat Kimia-Fisika
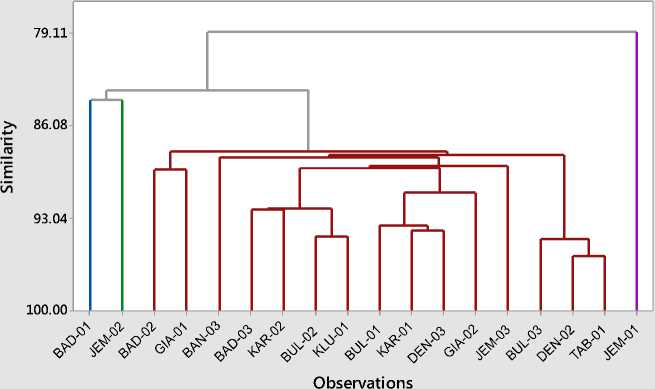
Fig. 6. Dendogram profile of diversity based on physico-chemical properties of fruit of 18 (eighteen) “Jeruk Bali” cultivars in Bali
CONCLUSIONS
RAPD analysis results at 53% similarity level are grouped into 5 groups. The first group was only one cultivar, the second group consisted of 13 cultivars, the third and fourth groups were only one cultivar, while the fifth group consisted of two cultivars. The analysis of the diversity between cultivars based on the combination of physical and chemical properties of the fruit with hierarchy method on similarity level about 85% in a group is obtained by 4 (four) groups. Groupings by combination of physical and chemical properties of the fruit are not synchronized in their entirety with dendograms based on their genetic diversity. This illustrates the physicochemical properties of “Jeruk Bali”fruit in general is not fully genetical expressed, but also influenced by conditions of environmental growth. Most cultivars were observed highly preferred by consumers,
but three less preferred cultivars namely “Jerungga” Bangli (BAN-03), “Juuk Bali”
Jembrana (JEM-03) and “Jeruti”
Karangasem (KAR-02).
REFERENCES
AOAC (Association of Official Analytical Chemists). (2005). Official Methods of Analysis. Ed 18th. Washington DC: AOAC International.
Ara, N., Bashar, M.K., Kalim, U. M.D., Khalequzzaman, K.M. (2008).
Evaluation of pummelo, Citrus grandis L. cultivars in northern area of Bangladesh. J Agric Res, 46: 65-75.
Azrai, M. (2005). Pemanfaatan Markah Molekuler dalam Proses Seleksi Pemuliaan Tanaman Balai Penelitian Tanaman Serealia, Maros. J. AgroBiogen, 1(1):26-37
Chang, C. C., Yang, M. H., Wen, H. M., & Chern, J. C. (2002). Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods, J. Food Drug Anal.
Dhakshanamoorthy, D., Selvaraj, R., Chidambaram, A. L. A. (2010). Induced mutagenesis in Jatropha curcas L. using gamma rays and detection of DNA polymorphism through RAPD marker. Cell R. Biologies 2011; 334:2430. doi:10.1016/j.crvi.2010.11.004
Direktorat Tanaman Buah. (2012). Budidaya Jeruk Besar (Citrus maxima L.). Jakarta: Direktorat Tanaman Buah, Direktorat Jenderal Bina Produksi Hortikultura.
Doyle, J. J., & Doyle, J. L. (1990). Isolation of plant DNA from fresh tissue. Focus, 12: 13– 15.
Febrianti, N., & Sari, F. J. (2016). Kadar Flavonoid Total Berbagai Jenis Buah Tropis Indonesia. Prosiding Symbion (Symposium on Biology Education), Prodi Pendidikan Biologi, FKIP, Universitas Ahmad Dahlan, 607- 612
Fernandez, M.R., Pearse, P. G., Holzgang, G., & Hughes, G.R. (2002). Fusarium head blight in barley in Saskatchewan in 2001. Can. Plant Dis. Surv. 82:32-33.
Hamilton, K.N., Ashmore., S. E., & Drew, R.A. (2008). Morphological
Characterization of Seeds of Three Australian Wild Citrus Species (Rutaceae): Citrus australasica F.
Muell., C. inodora F.M. Bailey and C. garrawayi F.M. Bailey . Genet Resources Crop Evol 55: 683-693.
Johnson, R. A & Wichern, D. W. (2007). Applied Multivariate Statistical Analysis, 6th edition. New Jersey:
Pearson Prentice Hall.
Lado, J., Rodrigo, M. J., & Zacarías, L. (2014). Maturity indicators and citrus fruit quality. Stewart Postharvest Review (2014), 2:2
www.stewartpostharvest.com. ISSN: 1745-9656 [cited 2014 Feb. 16].
Mahardika, I. B. K., & Susanto, S. (2003). Perubahan Kualitas Buah Beberapa Kultivar Jeruk Besar selama Periode Pematangan. Hayati. 10:106-109.
Niyomdham, C. (1992). Citrus maxima (Burm.) Merr. Di dalam: Verheij EWM and Coronel E, editor. Edible Fruits and Nuts. Plant Resources of South-East Asia. 2. Bogor : Prosea Foundation. hlm 128-131.
Paudyal, K.P., & Haq, N. (2007). Variation of pomelo (Citrus grandis (L.) Osbeck) in Nepal and participatory selection of strains for further improvement. Agroforest Syst, 72:195-204.
Pichaiyongvongdee, S., & Haruenkit, R.
(2009). Investigation of limonoids, flavanon, total polyphenol content and
antioxidant activity in seven Thai pummelo cultivars. Kasetsart J (Nat Sci), 43:458-466.
Rahman, M. M., Rabbani, M. G., Khan, A. S. M. M. R., Ara, N., & Rahman, M.O. (2003). Study on physio-morphological characteristics of different local pummelo accessions. Pak J Biol Sci, 6:1430-1434.
Rai, I N., Wijana, G., & Semarajaya, C. G. A. (2008). Identifikasi Variabilitas Genetik Wani Bali (Mangifera caesia Jack.) dengan Analisis Penanda RAPD. J. Hort, 18(2):125-134.
Riaz, M., T. Zamir., N. Rashid., N. Jamil., S. Rizwan., Z. Masood., A. Mushtaq., H. Tareen., M. Khan & M. Ali. (2015). Comparative Study of Nutritional Quality of Orange (Citrus sinensis) at Different Maturity Stages in Relation to Significance for Human Health. American-Eurasian Journal of Toxicological Sciences, 7 (4): 209-213.
Rout, G. R., Sunil, K., SenaPati, S., &
Aparajita. (2010). Study of Relationships among Twelve
Phyllanthus Species with the Use of Molecular Markers. Czech J. Genet. Plant Breed., 46, (3): 135–141.
Rukmana, R. (2009). Jeruk Besar; Potensi dan Prospeknya. Edisi ke 5. Kanisius. Yogyakarta.
Setiawan, A. I., & Sunarjono, H. (2003). Jeruk Besar Pembudidayaan di Pot dan di Kebun. Penebar Swadaya. Jakarta. 111 h.
Singh,S. K., Singh, I. P., Singh, A., Parthasarathy, VA. & Vinoth S. (2015). Pummelo [Citrus grandis (L.) Osbeck] Diversity in India. Indian J. Plant Genet. Resour, 28(1): 44-49
Somantri, I. H, Hasanah, M., & Kurniawan, H. (2008). Teknik Konservasi Ex-Situ, Rejuvenasi, Karakterisasi, Evaluasi, Dokumentasi, dan Pemanfaatan Plasma Nutfah.
http://indoplasma.or.id/artikel/artikel_2 005_teknik_konservasi.htm. [cited
2009 Agu.18 ].
Uzun, A., Gulsen, O., Yesiloglu, T., Aka-Kacarz Y., & Tuczu O. (2010).
Distinguishing grapefruit and pummelo accessions using ISSR markers. Czech J Genet Plant Breed. 46 (4):170–177.
Wagiyono. (2003). Menguji Kesukaan Secara Organoleptik. Jakarta: Bagian Proyek Pengembangan Kurikulum Direktorat Pendidikan Menengah Kejuruan Direktorat Jenderal Pendidikan Dasar dan Menengah Departemen Pendidikan Nasional.
Williams, J. G. K., Kubelik A .R., Livak, K.J., Rafalski, J.A., & Tingey, S.V. (1990). DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res, 18: 65316535.
ASIA OCEANIA BIOSCIENCE AND BIOTECHNOLOGY • 59
Discussion and feedback