POTENTIAL UTILIZATION IN DYE DECOLORIZATION OF SPENT MUSHROOM SUBSTRATES
on
Short communication
POTENTIAL UTILIZATION IN DYE DECOLORIZATION OF SPENT MUSHROOM
SUBSTRATES
Hee-Wan Kang1
1Graduate School of Future Convergence Technology, Hankyong National University, Ansung 456-749, Korea
*Corresponding author: Email: kanghw2@hknu.ac.kr
ABSTRACT
Pleurotus ostreatus, P. eryngii, and Flammulina velutipes are main edible mushrooms that account for over 89% of total mushroom production in Korea. Of them, cultivation of P. eryngii is 25%. About 2.5 million tons of spent mushroom substrate (SMS) is produced each year in Korea. It is either used as garden fertilizer or deposited in landfills, which pollutes the environment. This study was carried out to extract the lignocellulolytic enzymes amylase (EC 3.2.1.1), cellulase (EC 3.2.1.4), laccase (EC 1.10.3.2), and xylanase (EC 3.2.1.8) were efficiently extracted from spent mushroom substrate (SMS) of edible mushrooms. laccase activity were highest in extracts from the P. eryngii SMS, with values of 8.01 U/g. The synthetic dyes remazol brilliant blue R and Congo red were decolorized over 88 and 93% by the water extract of P. eryngii SMS within 120 min, and the extract’s decolorization ability was compared to commercial laccase. Furthermore, the SMS extract was applied to decolorize textile mill wastewater dye for its industrial application.
Keywords: Decolorization, Spent mushroom substrate, Water extract
INTRODUCTION
The mushrooms are mostly produced on a commercial scale using automated systems, and their culture substrates include numerous lignocellulosic wastes, including corncobs, sugarcane wastes, cottonseed hull, cotton and beet pulp, and sawdust supplemented with rice bran in vinyl bags and bottles. Spent mushroom substrate (SMS) weighing approximately 2.5 million tons is estimated to be produced yearly by mushroom farms in Korea. Because this massive amount of SMS is unsuitable for reuse in mushroom production, it is either used as garden fertilizer or deposited in landfills, which pollutes the environment. SMS is composed of fungal
mycelia, extracellular enzymes secreted from mushrooms for degrading substrates, and unused lignocellulosic substrates. SMS has been used for producing value-added products such as biogas (Bisaria et. al, 1990) and bulk enzymes (Kumaran et al., 1997; Singh et al., 2003), for bioconversion into organic fertilizer (Soechtig and Grabbe, 1995), for use as animal feed supplements (Vikineswary et al., 1997), and for degrading pentachlorophenol (PCP) (Chiu et al., 1998). These uses benefit human health and the environment because an agricultural waste forms the raw material for novel processes. Mushrooms can enzymatically degrade diverse substrates containing lignin, hemicellulose, and cellulose
ISSN ONLINE: 9 772303 337 008
into soluble compounds of low molecular weight. These soluble compounds are then absorbed by the fungal hyphae by a process called nutritive absorption. Three major groups of enzymes involved in breaking down lignin and cellulosic agro-wastes are cellulases, xylanases, and ligninases. Practically, lignin degrading enzymes such as laccases have been used to the decolorize the industrial synthetic dyes such as remazol brilliant blue R (RBBR), Congo red, and indigo (Suwannawon et al., 2010). In this study, water extract from SMSs of edible mushrooms was examined for potential usefulness to decolorize synthetic dyes.
Bulk enzymes from spent mushroom substrates
Spent mushroom substrates of Pleurotus cornucopiae, Pleurotus ostreatus, Pleurotus eryngii, Hericium erinaceum, Lyophyllum ulmarium, Agrocybe cylindracea, Lentinus lepideus and Flammulina velvtipes were collected immediately after harvesting mushrooms. Bulk enzymes were extracted using 50 mM sodium citrate buffer (pH 4.5). The SMS-buffer mixtures were incubated with shaking at 200 rpm for 2 h at room temperature. The sample was filtrated through miracloth (pore size:22-25 micrometer) and then centrifuged at 10000 × g at 4°C for 15 min. The supernatant was used for enzymatic assay of the lignocellulolytic enzymes amylase (EC 3.2.1.1), cellulase (EC 3.2.1.4), laccase (EC 1.10.3.2), and xylanase (EC 3.2.1.8). Laccase activity was assayed by measuring the oxidation of 2, 2ʹ-azinobis [3-
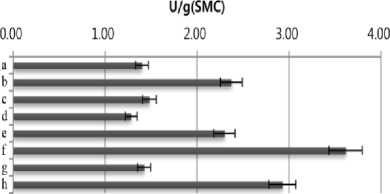
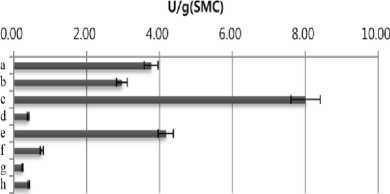
ethylbenzothiazolone-6-sulfonic acid] (ABTS). SMS extract (10 µL) was mixed with 90 µL of 50 mM sodium acetate buffer (pH 4.5) containing 1 mM ABTS and then reacted at room temperature. The reaction was stopped by adding 100 µL of 20% (w/v) trichloroacetic acid (TCA). Oxidation of ABTS was monitored at 420 nm on a spectrophotometer. One unit of enzyme activity was defined as the amount of enzyme required to oxidize 1 μmol/min of the substrate under the assay conditions. As shown in Fig. 1, highest amylase (3.81 U/g) and cellulase (3.3 U/g ) activities were detected in SMS extract of Agrocybe cylindracea. Flammulina velvtipes showed the highest xylanase activity (152 U/g). On the other hand, the highest activity of laccase (8.01 U/g) was observed in water extract of P. eryngii SMS .
Dye decolorization using water extract from SMS
The following experiment focused on practical application in dye decolonization using SMS extract of P. eryngii that showed the highest laccase activity. The water extract from in SMS extract of P. eryngii was subjected to confirm decolonization of different dyes including Bromophenol blue (605 nm), Congo red (532 nm), Coomassie brillient blue (627 nm), Crystal violet (589 nm), Methylen blue (587 nm), Ramazol brillient blue R (597 nm). In addition, Rit-blue (510 nm), and Rit-red (603 nm), which have been used in texture dyes was used in this study.
-
A. Amylase C. Laccase
-
B. Celluase D. Xylanase
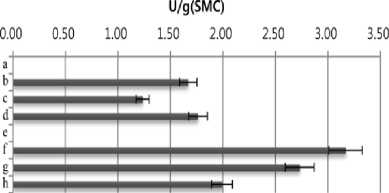
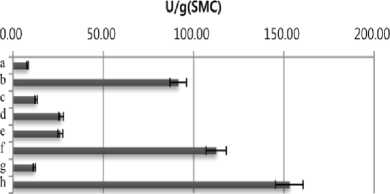
Fig. 1. Productivity of enzymes in spent mushroom substrates (SMSs) of different mushroom species. a, Pleurotus cornucopiae;b, Pleurotus ostreatus; c, Pleurotus eryngii; d, Hericium erinaceum; e, Lyophyllum ulmarium; f, Agrocybe cylindracea; g, Lentinus lepideus; h, Flammulina velvtipes. The results are mean (±S.D.) of three replicate samples.
The decolorization percentage was calculated as follows: Decolorization percentage (%) = (A0 - A)/A0 × 100, where A0 is the dye absorbance of the control, and A is the dye absorbance of the test sample. Water extract P. eryngii SMS showed the highest decolorization activity over 93 and 88% in remazol brilliant blue R and Congo red among different commercial dyes after 24 hr of their treatments. The two industrial synthetic acidic dyes, RBBR and Congo red, represent anthraquinone and diazo dyes, respectively, and they were used as decolorization substrates to evaluate the dye-decolorization efficacy of laccase in the SMS extracts. Interestingly, SMS decolorized the diazo-like dye Congo red without any mediator, even though this dye is not a common substrate for
the laccase catalytic reaction (Wang et al., 2012).
Laccase is the most widely distributed of all of the large blue copper-containing proteins present in diverse higher plants and fungi. Laccase belongs to a broad group of enzymes called polyphenol oxidases, which contain copper atoms in the catalytic center and are usually called multicopper oxidases. Laccases are similar to other phenol-oxidizing enzymes, which preferably polymerize lignin by coupling with the phenoxy radicals produced by oxidation of lignin phenolic groups. Fungal laccases have widespread applications, ranging from effluent decolorization and detoxification to pulp bleaching, removal of phenolics from wines, organic synthesis, manufacture of biosensors, synthesis of complex medical compounds, and
ISSN ONLINE: 9 772303 337 008
dye-transfer blocking functions in detergents and washing powders (Polizeli et al., 2005). In Fig. 3(A), water extract of different Pleurotus spp.SMS was subjected to decolorize Ramazol
brillient blue R. Of them, water extract of P. eryngii SMS showed powerful decolorization efficacy.
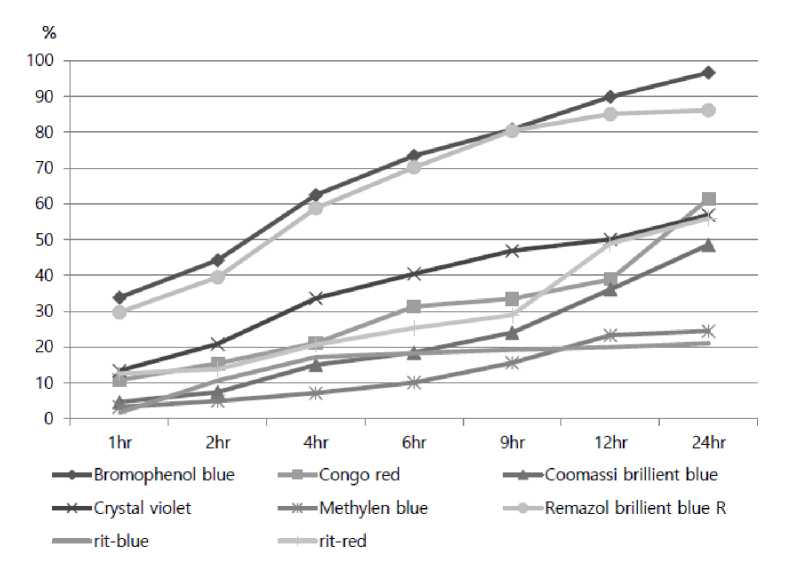
Fig 2. Effect of dye decolorization rate of different dyes by extract from Pleurotus eryngii SMS on time intervals. Each 10 ^ aliquot of the spent mushroom substrate extract was applied to the dye decolorization. Each dye decolorization was measured by using a spectrophotometer on wavelengths ranged from 500nm to 650 nm. The blank indicates the use of boiled SMS extract in reaction.
CONCLUSION
Most of the current processes used to treat dye wastewater are ineffective and uneconomical (Cooper, 1995; Stephen, 1995) because of their cost-consuming steps and limited applications. Therefore, the development of waste-treatment processes based on laccase appears to be an attractive solution because the products have the potential to degrade dyes with diverse
chemical structures (Abadull et al., 2000; Hou et al., 2004) including synthetic dyes used in today’s industries (Munoz et al., 1997; Rodríguez et al., 2004; Rodríguez et al., 2005). Fig. 3(C) shows dye decolorization of textile mill wastewater after 24h of SMS extract treatment of P. eringii. The results suggest the SMS of P. eringii that retains high laccase activity is can potentially be utilized in biological dye decolorization.
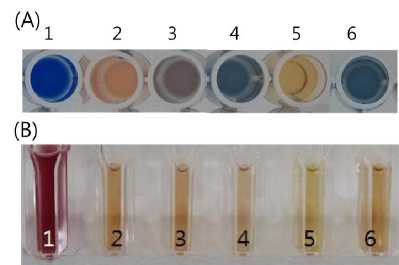
Fig. 3. Dye decolorization by water extract of spent mushroom substrates. A: dye decolonization of Ramazol brillient blue R by 16, distilled water, Laccase 1mg/ml (Sigma ), 100ug/1ml (Sigma), Water extract (10 μL)of Pleurotus cornucopiae SMS, Water extract (10μL) of P. eryngii SMS and Water extract (10μL) of P. ostreatus SMS. B: dye decolorization of textile mill wastewater using water extract of P. eryngii SMS;1-6 boiled water extract (50 μL) of P. eryngii SMS, Water extract (10 μL) of P. eryngii SMS, Water extract (20 μL) of P. eryngii SMSE, 30 μL of P. eryngii SMS, 40 μL of P. eryngii SMS, Water extract (50 μL) of P. eryngii SMS.
Fungal laccase genes have been overexpressed in different host systems such as yeast and Aspergillus niger (Lu et al., 2009; Rodríguez et al., 2006), but the lack of sufficient enzyme production is the biggest obstacle in the commercial application of laccases. Moreover, the purification of laccase requires several complicated steps that lead to substantial losses of the enzyme (Guillén et al., 1997; Han et al., 2005). In this study, the SMS that is agricultural waste was found to contain large amounts of lignin-degrading enzymes, and the crude enzymes could be used to decolorize dyes. Further study is required to optimize the purification system for these degradation enzymes to make purification inexpensive and suitable for industrial applications and
to determine how the crude enzymes can be stored for extended periods.
REFERENCES
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gübitz GM. Decolorization and detoxification of textile dyes with a laccase from Trametes hirsuta. Appl Environ Microbiol 2000;66:3357-3362.
Bisaria R, Vasudevan P, Bisaria VS. Utilization of spent agro-residues from mushroom cultivation for biogas production. Microbiol Biotechnol 1990;33:607–609.
Chiu SW, Ching ML, Fong KL, David M. Spent oyster mushroom substrate performs better than many mycelia in removing the biocide
pentachlorophenol. Mycological
research 1998;102:1553–1562.
Han MJ, Choi HT, Song HG. Purification and characterization of laccase from the white rot fungus Trametes versicolor. J Microbiol 2005;43:555-560.
Cooper P. Removing color from dye house waste water. Asian Textile J 1995;3:52-56.
Guillén F, Martínez AT, Martínez MJ. Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Current
Microbiology 1997;34:1-5.
Hou H, Zhou J, Wang J, Du C, Yan B. Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochemistry 2004;39:415– 1419.
Kumaran S, Sastry CA, Vikineswary S. Laccase, cellulase and xylanase
activities during the growth of
Pleurotus sajor-caju on sago hampas.
ISSN ONLINE: 9 772303 337 008
World Journal Microbiol Biotechnol 1997;13:43–49.
Lu L, Zhao M, Liang SC, Zhao LY, Li DB, Zhang BB. Production and synthetic dyes decolourization capacity of a recombinant laccase from Pichia pastoris. J Appl Microbiol 2009;107:1149–1156.
Munoz C, Guille F, Martinez AT, Martinez MJ. Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic
properties, and participation in activation of molecular oxygen and Mn21 oxidation. Applied and Evrironmental and Microbiology 1997;63:2166–2174
Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: properties and industrial applications. Appl Microb Biotech 2005;67(5):577-91.
Rodríguez CS, Hofer D, Sanromán MA, Gübitz GM. Production of laccase by Trametes hirsuta grown in an immersion bioreactor. Application to decolourisation of dyes from a leather factory. Engineering in Life Sciences 2004;4: 233-238.
Rodríguez CS, Sanromán M, Gübitz GM. Influence of redox mediators and metal ions on synthetic acid dye decolourization by crude laccase from Trametes hirsuta. Chemosphere 2005;58: 417-422.
Rodríguez CS, Toca Herrera JL. Industrial and biotechnological applications of laccases: A review. Biotechnology Advances 2006;24(5):500-513.
Singh AD, Abdullah N, Vikineswary S. Optimization of extraction of bulk enzymes from spent mushroom compost. Chemical Technology and Biotechnology 2003;78(7):743–752.
Soechtig H, Grabbe K. The production and utilization of organic-mineral fertilizer from spent mushroom compost. Mushroom Science 1995;2:907–916.
Stephen JA. Electrooxidation of dyestuffs in waste waters. J Chemical Technol and Biotechnol 1995;62: 111-117.
Suwannawong P, Khammuang S, Sarnthima R. Decolorization of rhodamine B and congo red by partial purified laccase from Lentinus polychrous Lév. J Biochem Tech 2010; 3(2):182-186
Vikineswary S, Kumaran KS, Ling SK, Dinesh N, Shim Y.L. Solid substrate fermentation of agroresidues for value added products: the Malaysian
experience. Global Environmental Biotechnology 1997;301-305.
Wang W, Zhang Z, Ni H, Yang X, Li Q, Ni L. Decolorization of industrial synthetic dyes using engineered Pseudomonas putida cells with surface-immobilized bacterial laccase. Microbial Cell Factories 2012; 11:7514.
96 • asia oceania bioscience and biotechnology consortium
Discussion and feedback