PHYTOREMEDIATION OF MERCURY CONTAMINATED SOILS IN A SMALL SCALE ARTISANAL GOLD MINING REGION OF INDONESIA
on
PHYTOREMEDIATION OF MERCURY CONTAMINATED SOILS IN A SMALL SCALE ARTISANAL GOLD MINING REGION OF INDONESIA
Kokyo Oh1, Sachiko Takahi2, Sri Wedhastri4, Hardita Librasanti Sudarmawan 5, Retno Rosariastuti6 and Irfan Dwidya Prijambada3*
-
1.Center for Environmental Science in Saitama
-
2Faculty of Education and Human Studies of Akita University 3Graduate School of Biotechnology of Gadjah Mada University 4Graduate School of Biotechnology of Gadjah Mada University
-
5Dept. of Agricultural Microbiology, Fac. of Agriculture of Gadjah Mada University 6Sebelas Maret Universty
*Corresponding author : irfandp@faperta.ugm.ac.id
ABSTRACT
In the small gold mining regions of Indonesia, the tailings or waste water containing mercury commonly may be released into agricultural lands resultimg soil contamination. Phytoremediation is a low-cost and environmental friendly alternative to traditional techniques such as soil heating, soil removal, and soil washing. In this study, a sweet sorghum combined with the inoculation of a rhizobacteria, Agrobacterium tumefaciens, was tested in a field experiment with mercury contaminated soil from a small scale gold mining. Plant growth, uptake and accumulation of mercury by the sweet sorghum, and effects of the Agrobacterium tumefaciens inoculation on mercury accumulation were investigated. The average of mercury content in the soil was 3.76 mg/kg. The results showed that the sorghum grew well, and the inoculation of Agrobacterium tumefaciens promoted the plant growth, but did not increased the mercury concentration in both root and stem parts of the sorghum. The accumulation of mercury was 6.2^/plant for sorghum without Agrobacterium tumefaciens, and 14.0^/plant for sorghum with Agrobacterium tumefaciens. It was estimated that the phytoremediation efficiency of mercury was 414 and 934 mg/ha for sweet sorghum without Agrobacterium tumefaciens inoculation and with Agrobacterium tumefaciens inoculation, respectively.
Keywords: Soil contamination, mercury, phytoremediation, sweet sorghum, Indonesia
INTRODUCTION
Mercury is a global environmental pollutant presented in soil, water, atmosphere and biota, and one of the “priority hazardous substances” listed by the Agency for Toxic Substances and disease registry (ATSDR) because of its toxicity, mobility, and long residence time in the atmosphere (Wang et al., 2012). Mercury pollution is a serious threat to human health and the environment. Mercury exposure at high levels can harm the brain, heart, kidneys, lungs, and immune system of people of all ages. The most serious effect is damage to the central nervous system, especially in fetuses and young children (CPA, 2010).
Mercury enters environment through natural process and human activities. Natural
processes generally constitute the rock weathering, volcanic activities and geothermal activities (Nriagu et al., 2003). The average background concentration of mercury in soils is averagely 0.06 mg/kg with a general range from 0.03 to 0.1 mg/kg (Wang et al., 2012). The anthropogenic sources of mercury can be attributed as coal combustion, waste incineration, metal refining and manufacturing (Wang et al., 2012; UNEP, 2013). According to UNEP (2013), it is estimated that the global emissions to air from anthropogenic sources is 1960 tonnes in 2010, responsible for about 30% of annual total emissions of mercury to air. The artisanal and small-scale gold mining (ASGM) and coal burning is the largest components of anthropogenic emissions, followed by the production of ferrous and non-ferrous metals,
and cement production. Annual emissions from ASGM are estimated at 727 tonnes, responsible for the largest sector accounting for over 35% of total anthropogenic emissions. Asia is the main source region of mercury emissions to air, and East and Southeast Asia account for about 40% of the global total (UNEP, 2013).
Indonesia is one of the major location for ASGM, where there are 731 ASGM sites throughout the country (Muddarisna et al., 2013). ASGM are causing environmental pollution because of the widespread use of mercury in the gold extraction processes. After the gold-containing ore or silt is thoroughly grinded, mercury is added to the mixture to create a gold amalgam. Subsequent burning of the amalgam concentrates the gold into a pellet but releases elemental mercury into the environment. There is a mercury loss to the environment through discharge of water used for grinding. The discharge of gold amalgamation tailings to agricultural lands also causes soil contamination. The contamination of soil with mercury has led to environmental concerns, as mercury can readily be absorbed by plants and be accumulated in the human body through the food chain. It has been well known that crops grown in mercury contaminated soil have an elevated total mercury content in their tissues (Qian et al.2009; Wang et al., 2012). Studies also clearly show that mercury contamination of soils result in an elevated exposure of mercury to local residents and pose a direct threat to the health of these people (Wang et al., 2012). Therefore, there is a great need to develop suitable methods for remediation of mercury contaminated soils.
During the past decade, phytoremediation, the use of green plants and their associated microbiota for the in situ treatment of contaminated sites has received increasing attention as a cost-effective and ecofriendly technology (Salt et al. 1998; Ahmadpour et al. 2012; Oh et al., 2013). Phytoremediation to date indicates that it is applicable to a wide range of inorganic pollutants such as Cd, Pb, Cu, Ni, Hg, and As,
and organic pollutants such as pesticides and chlorinated solvents (Oh et al., 2013).
The phytoremediation usually has a low remediation rate, and thus generally a longer period (usually over 3 or 5 years) is needed comparing to other physicochemical methods. To improve the phytoremediation rate, studies have focused on hyper accumulator plants, which is capable of accumulating potentially phytotoxic elements to concentrations much higher than the normal plants growing in the same environment (Salt et al. 1998; Oh et al., 2013). However, most of these hyperaccumulator plants usually have a low annual biomass production, which tends to limit their remediation ability. Moreover, as they have very high heavy metal concentration, after harvested the plants usually need incineration treatment. In this way, the conventional phytoremediation needs cost year by year, and the owners of the contaminated sites have no income during the remediation period, which has been limited the practical application of phytoremediation (Oh et al., 2013; 2014).
In recent years, other economic plants are receiving increasingly attention as they can both remediate contaminated soils, and produce valuable biomass, through which the practical application of phytoremediation can be promoted and the soil resources can be protected (Chintakovid et al., 2008; Oh et al. 2014).So far, high biomass crops such as Indian mustard (Brassica juncea), maize (Zea mays), sunflower (Helianthus annus), and sweet sorghum (Sorghum bicolor var. saccharatum) have been demonstrated to be able to remediated various heavy metals (Utami et al., 2013; ). To improve the remediation rate, studies on means for enhancing phytoremdiation have been extensively carried out. The measures for enhanced phytoremediation mainly include application of chelating agents, effective bacteria, and genetic plants.
Soil from gold mining areas generally has a high proportion of bioavailable mercury because of the release of mercury into the soil as a soluble form. Therefore, phytoremdiation
ISSN ONLINE: 9 772303 337 008
should be a suitable choice for these soils or tailings.
The objectives of this study are to determine the potential of a sweet sorghum for phytoremediation of mercury contaminated soil in the small scale gold mining area in Indonesia, and the effects of inoculation of a rhizobacteria, Agrobacterium tumefacian.
MATERALS AND METHODS
Study area
This study was carried out in the field owned by Mr. Sutaryo where the soil was contaminated by discharge of small-scale gold amalgamation tailings containing mercury. The site is located in Nglenggong, Jendi, Selogiri, Wonogiri, Jawa Tengah, Indonesia (07°47´51"S, 110°52´21"E), with an elevation of 498 feet.
Soil preparation and fertilization
Field experiment was conducted from June to November, 2013. The soil had an initial total mercury content of 3.8 mg/kg. When experiment started, the soil was mixed evenly, and was applied fertilizers of nitrogen, phosphate and potassium with the fertilization rates as shown in form1. The experimental field was divided into nine blocks. Two treatments with three replications were designed. The two treatments were sweet sorghum plantation (TN) and sweet sorghum plantation with bacteria inoculation, respectively.
Sweet sorghum plantation and rhizo-bacteria inoculation
Sweet sorghum variety KCS105 was used in this study. The seeds of the KCS105 were germinated at room temperature for 1 week in petri dishes. Then, the sorghum seedlings of similar size and appearance were used to transplant to the experimental field, with a plantation density of 667,00 plant/ha.
A rhizobacterial isolate (I29 Isolate), Agrobacterium tumefaciens, which was proved to be able to enhance chromium uptake by maize, were used in this study. As described by
Utami et al.(2013), the Chromium uptake enhancing Agrobacterium tumefaciens was cultured in liquid LB medium for 24 hours until its cell concentration reached 108 CFU/ml. The inoculation of the Agrobacterium tumefaciens was done when the sorghum seedlings were transplanted by pouring the bacterial cultural solution to the soil. Sweet sorghum was harvested 12 weeks (about three months) after transplantation. The plant height above the ground in each plot was measured once a week. After harvest, shoots and roots were separated, washed, weighted and oven dried at 40°C for 48 hours for determining the dry biomass of the plants and the mercury content.
|
Table 1. Fertilization experimental field |
rates |
in the | |
|
Fertilizers |
Fertilization rate (kg/ha) | ||
|
Urea |
257 | ||
|
Rock Phosphate |
511 | ||
|
SP36 |
85 | ||
|
K Organic |
410 | ||
|
POP |
900 | ||
Mercury analysis
Oven died plants were cut into small pieces and ground, then, the powdered plant tissues were dissolved in 5ml of dilute acid mixture (60% HClO4; 65% HNO3, 1:1 v/v) in flask. Mercury content in plant and soil were analyzed using a FIMS 100CVAAS from Perkin Elmer.
RESULTS AND DISCUSSION
Growth of sweet sorghum
Plant height within 12 weeks is shown in Fig.1. Plant height both for TN and TNB increased with time, indicating that the sorghum
grew well in the mercury contaminated soil. Plant of TNB treatment showed a higher plant height from the 2nd week than that of TN treatment. The plant in the TNB treatment had 107 cm height at the 12th week, while the plant of the TN treatment had a height of 93 cm. This indicated that Agrobacterium tumefaciens inoculation to sweet sorghum improved 15% to the plant height.
The total dry weight biomass in TN and TNB treatments were 5.0 and 8.0 g/plant, respectively, indicating that Agrobacterium tumefaciens inoculation improved 40% of the biomass production (Fig.2). The root dry weight biomass in TN and TNB treatments were 1.1 and 2.7 g/plant, while the shoot dry weight biomass were 3.8 and 5.3 g/plant, respectively, as shown in Fig.2. In contrast to the control without inoculation (TN), there was a 39% increase rate for shoot, and a 250% increase rate for root, respectively.
The results indicated that sweet sorghum could survive and grow well in the mercury contaminated soil, and its growth was promoted with Agrobacterium tumefaciens inoculation. As the root in TNB treatment had dry weight biomass about 2.5 times of that in TN treatment,
the promotion of Agrobacterium tumefaciens inoculation to sorghum plant growth is possibly through promotion to the root growth.
Agrobacterium tumefaciens is a soil-borne bacterium that, in nature, is capable of inserting a defined fragment of its DNA into the genome of dicotyledonous plants, and can cause disease in more than 90 families of dicotyledonous plants (Valentine, 2003; Hao et al. 2012). However, not all Agrobacterium tumefaciens were pathogenic, some were beneficial to assist rhizobia nodulating with legumes and promote plant growth (De Lajudie 1999; Hao et al. 2012).
The results in this study showed that the Agrobacterium tumefaciens I29Isolate was a plant growth promoting endophytic bacterium. Our results were supported by the findings of Hao et al. (2012), who reported that Agrobacterium tumefaciens (CCNWGS0286) displayed a significant increase in biomass production over that without inoculation in a zinc contaminated environment, and also found that phytohormones, were the dominant factor in enhancing plant growth in contaminated soil.
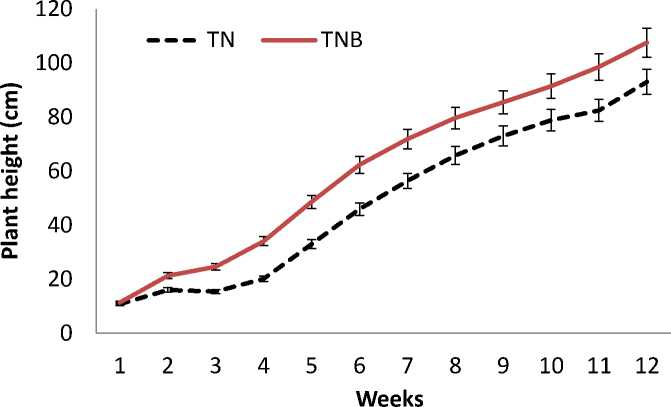
Fig.1. Plant height of sweet sorghum with and without Agrobacterium tumefaciens inoculation.
Values are means±SE.
ISSN ONLINE: 9 772303 337 008
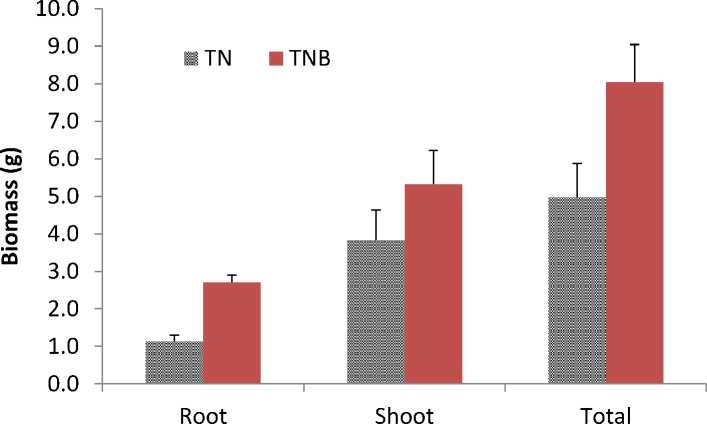
Fig.2. Biomass of individual sweet sorghum plant with and without Agrobacterium tumefaciens inoculation. Values are means±SE.
Table 2. Mercury concentration of sweet sorghum and the shoot/root concentration ratio (means±SE)
|
Treatments |
Root |
Shoot |
Whole plant |
Shoot/root Ratio |
|
TN |
2.62±0.29 |
1.19±0.24 |
1.60±0.28 |
0.45±0.07 |
|
TNB |
3.10±0.27 |
1.19±0.40 |
1.72±0.28 |
0.44±0.19 |
Mercury concentration in the sweet sorghum
Mercury concentrations in different parts of the plants are shown in Table 2. As shown, for TN treatment, the mercury concentration in root, shoot and whole plant were 2.62, 1.19 and 1.62 mg/kg, respectively. For TNB treatment, the mercury concentration in roots, shoots and whole plants were 3.10, 1.19 and 1.72 mg/kg, respectively. The concentration in roots was more than 2 times that in shoots for both TN and TNB treatments. The mercury concentration in the sweet sorghum in this study reached a very high level compared to other common crops, although it was lower than some wild plant species in Indonesia such as Lindernia crustacea (L.) F., and Paspalum conjugatum L. reported by Muddarisna et al.(2013). It was also found that the shoot/root concentration ratio was much higher than some of phytoremediation potential plants such as
Indian mustard and Chinese brake fern (Su et al. 2007), indicating that the sweet sorghum possibly had a higher mercury translocation potential from root to shoot.
However, no promotion effect on mercury concentration of sweet sorghum was found with Agrobacterium tumefaciens inoculation in the current field experiment. This result confirmed with the findings by Prijiabada et al.(1999) that inoculation of Brassica juncea with fluorescent peseudomonads did not promoted heavy metal uptake ability of the plant. However, the result was different from the observation with a pot cultural experiment by Utami et al. (2013), who reported that inoculation of chromium enhancing rhizobacteria increased mercury content in sweet sorghum up to about 6 times, but did not promoted plant growth.
Phytoremediation potential of the sweet sorghum
Phytoremediation capacity of plants to soil heavy metals is generally decided by the heavy metal accumulation amount of plants, which can be calculated by multiplying plant weight with its mercury content. The mean values of accumulation amount of individual sweet sorghum are shown in table 3.
As shown in table 3, the mean values of mercury accumulation was 6.2^/plant for sweet sorghum without Agrobacterium tumefaciens inoculation, and 14.0^ plant for sweet sorghum with Agrobacterium tumefaciens inoculation, indicating that Agrobacterium tumefaciens inoculation promoted the accumulation amount of sweet sorghum. As there no difference in mercury content of sweet sorghum between TN and TNB (Table 1.), the increase of the accumulation amount was contributed by promotion of plant growth with Agrobacterium tumefaciens inoculation. With AT inoculation, it was observed a big increase of mercury accumulation amount in root, but a small increase in shoot. This indicates that Agrobacterium tumefaciens inoculation mainly promoted mercury accumulation in root, and therefore resulted in a smaller shoot/root ratio compared with those without Agrobacterium tumefaciens inoculation. The accumulation amount of the
sweet sorghum in this study compared with the results of Utami et al.(2012), who reported that the mercury accumulation amount of sweet sorghum is 2.19-8.57 for rhizobacteria inoculation, and 0.66-3.54 for control.
According to the plantation density of sweet sorghum in this study (66,700 plants per ha), it was estimated that the phytoremediation efficiency (phytoextraction yield) of mercury was 414 mg/ha for sorghum without Agrobacterium tumefaciens, and 934 mg/ha for sorghum with Agrobacterium tumefaciens inoculation as shown in Table 4. Compared without Agrobacterium tumefaciens inoculation, the Agrobacterium tumefaciens inoculation promoted the phytoremediation efficiency at increasing rates of 2.3 times for the whole plant, 1.6 times for shoot, and 3.2 times for root, respectively. Although plants generally have low mercury uptake as mercury usually has a low availability in the soils, the present study found that sweet sorghum could accumulate mercury at a considerable level. The phytoremediation efficiency of sweet sorghum obtained in the present study compared with the results by Rodriguez et al.(2005) who reported that the best mercury phytoextraction yield is obtained for barley reaching up to 719 mg/ha.
Table 3. Mean values (±SE) of mercury accumulation of individual sweet sorghum plant (^/plant)
|
Treatments |
Root |
Shoot |
Whole plant |
Shoot/root Ratio |
|
TN |
2.70±0.68 |
3.50±1.20 |
6.20±1.80 |
1.45±0.34 |
|
TNB |
8.50±2.20 |
5.50±0.50 |
14.0±2.40 |
0.80±0.18 |
Table 4. Phytoremediation efficiency of sweet sorghum (mg/ha, values are means±SE).
|
Treatments |
Root |
Shoot |
Whole plant |
|
TN |
180±45 |
233±80 |
414±120 |
|
TNB |
567±147 |
367±33 |
934±160 |
ISSN ONLINE: 9 772303 337 008
It is a reasonable way to regard the contaminated soils as a potentially recoverable resource for both remediation and utilization. So far, many studies on phytoremediation have mainly focused on accumulator or hyperaccumulating plants. Actually, it is difficult to convince the local people, especially in developing countries, to grow special accumulators in the contaminated fields for the sole purpose of soil remediation unless they could obtain financial income. The present study showed that the sweet sorghum had high potential for phytoremediation. As sweet sorghum can be used feedstock for biofuel, it could bring income to the remediators or the owners of the contaminated sites. Considering the economic effects of the biofuel plants, the sweet sorghum were possibly a promising way for both utilization and remediation of contaminated sites.
CONCLUSIONS
The experimental results and their discussions allow us to conclude that the sweet sorghum variety KCS105 could be considered a suitable candidate energy crop for phytoremediation of mercury contaminated soil. It was able to grow well in the mercury contaminated soil and had considerable mercury accumulation ability in root and shoot. The phytoremediation efficiency estimated in this experiment was approximately 414 mg/ha without Agrobacterium tumefaciens inoculation, and 934 mg/ha with Agrobacterium tumefaciens inoculation. The current field experiment also showed that the increase of mercury accumulation by the sweet sorghum with the Agrobacterium tumefaciens inoculation was not from the increase of mercury concentration in plant, but from the increase of biomass production. As sweet sorghum is also a biofuel crop, which can bring income for the owners of the
contaminated sites, thus, the present study primarily provided valuable data supporting the use of biofuel plants in phytoremediation of mercury contaminated soil.
ACKNOWLEDGEMENTS
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant-in-Aid for Scientific Research (B) Number 25301001.
REFERENCES
Ahmadpour P, Ahmadpour F, Mahmud TMM, Abdu A, Soleimani M, and Tayefeh FH. 2012. Phytoremediation of heavy metals: a green technology, African Journal of Biotechnology 11: 14036- 14043.
Chintakovid W, Visoottiviseth P, Khokiattiwong S, and
Lauengsuchonkul S. 2008. Potential of the hybrid marigolds for arsenic phytoremediation and income generation of remediators in Ron Phibun District, Thailand.
Chemosphere 70:1532-1537.
CPA (Climate and Pollution Agency), 2010. The Mercury Problem: Reducing and eliminating mercury pollution in Norway, pp. 1-12.
De Lajudie P, Willems A, and Nick G. 1999. Agrobacterium 526 bv. 1 strains isolated from nodules of tropical legumes. Systematic and applied microbiology 22:119-132.
Hao X, Xie P, Johnstone L, Miller SJ, Rensing C, Wei G. 2012. Genome sequence and mutational analysis of plant-growth-promoting bacterium Agrobacterium tumefaciens
CCNWGS0286 Isolated from a zinclead mine tailing. Appl Environ Microbiol. 78:5384-94.
Muddarisna N, Krisnayanti BD, Utami SR, Handayanto E. 2013.
Phytoremediation of mercury-contaminated soil using three wild
plant species and its effect on maize growth. Applied Ecology and Environmental Sciences 1: 27-32.
Nriagu J and Becker C.2003.Volcanic emissions of mercury to the atmosphere: global and regional
inventories. Sci. Total Environ.304: 3-12.
Oh K, Cao T, Li T, and Cheng H. 2014. Study on application of
phytoremediation technology in management and remediation of contaminated soils. Journal of Clean Energy Technology 2: 216-220.
Oh K, Li T, Cheng H, Hu X, He C, Yan L, Yonemochi S. 2013. Development of profitable phytoremediation of contaminated soils with biofuel crops. Journal of Environmental Protection 4: 58-64.
Qian J, Zhang L, Chen H, Hou M, Niu Y, Zu Z, and Liu H. 2009. Distribution of mercury pollution and its source in the soils and vegetables in Guilin area, China. Bull. Environ. Contam. Toxicol. 83:920-925.
Rodriguez L, Lopez-Bellido F J, Carnicer A, Recreo F, Tallos A, Monteagudo J M. 2005.Mercury Recovery from Soils by Phytoremediation. In: Lichtfouse E, Schwarzbauer J, Robert D, editors Environmental Chemistry p 197-204.
Salt CA, Smith RD, and Raskin I. 1998.Phytoremediation, Annual
Review of Plant Physiology and Plant Molecular Biology. 49: 643648.
Su Y, Han F, Shiyab S, Monts D L. Phytoextraction and accumulation of mercury in selected plant species grown in soil contaminated with different mercury compounds. WM’07 Conference, Tucson, AZ, February 25 - March 1, 2007.
UNEP, 2013. Global Mercury Assessment 2013: Sources, Emissions, Releases and Environmental Transport. UNEP Chemicals Branch, Geneva,
Switzerland
Utami D, Takahi S, and Prijambada I D. 2013. Mercury acculation in gold mine tailing by sweetsorghum inoculated with chromium uptake enhancing rhizobacteria.
International Journal of Biosciences and Biotechnology 1: 86-90.
Valentine L.2003. Agrobacterium
tumefaciens and the Plant: The David and Goliath of Modern Genetics. Plant Physiology 133: 948955.
Wang J, Feng X, Anderson CWN, Xing Y and Shang L. 2012. Remediation of mercury contaminated sites-a review. Journal of Hazardous Materials 221222:1-18.
ASIA OCEANIA BIOSCIENCES AND BIOTECHNOLOGY CONSORTIUM • 21
Discussion and feedback