DETECTION OF CITRUS VEIN PHLOEM DEGENERATION (CVPD) DISEASE BY POLYMERASE CHAIN REACTION (PCR) AND PROTEIN ANALYSIS USING SDS PAGE (A Review)
on
ARTICLE
DETECTION OF CITRUS VEIN PHLOEM DEGENERATION (CVPD) DISEASE BY POLYMERASE CHAIN REACTION (PCR) AND PROTEIN ANALYSIS USING SDS PAGE
(A Review)
I Gede Putu Wirawan1 and Ketut Srie Marhaeni Julyasih2 1Agriculture Faculty Udayana University Bali,Indonesia 2Agriculture Faculty UPN”Veteran” East Java-Surabaya, Indonesia *Corresponding author : igpwirawan@yahoo.com
ABSTRACT
Citrus Vein Phloem degeneration (CVPD) is the most important disease and a major cause of yield loss citrus plantations in almost all countries, especially Asia and Africa. CVPD disease is caused by gram negative bacteria, Liberobacter asiaticum L. Pathogens can not be cultured in vitro, but can be detected by PCR using 16S rDNA fragment as a primer and by electron microscopy. The use of PCR using specific primer pair can detect infected plants more accurately. Citrus plant that infected by CVPD disease contain a very specific protein that produced by the pathogen. The specific protein molecules can be detected by comparing the protein bands of infected citrus plants and the healthy one which have molecular weight of approximately 16 kDa and 66 kDa, whereas in healthy citrus plant that protein molecule was not found.
Keywords : Citrus Vein Phloem Degeneration, Polymerase Chain Reaction, SDS-PAGE
INTRODUCTION
Citrus Vein Phloem degeneration (CVPD) is the most important disease and a major cause of yield loss citrus plantations in almost all countries, especially Asia and Africa. In 1965 in Africa reduced citrus crops from CVPD diseases between 30100%. Previous attacks occurred in 19321936 and in 1939-1946. Vietnam, especially in the Mekong Delta, 70-79% of the crop has been infected and Vinh Long Province and Can Tho reduced crop yields 42%. Damage to crops in the Philippines is estimated seven million trees in 1962 and 1971, wiping out more than one million trees in one area. In Thailand damage of plants more than 95%, while in Indonesia approximately three million damaged crops between the years 1960-1970 (Julyasih, 2009).
Typical symptoms of the disease are the leaves become yellow, bones of leaves dark green, the leaves become more rigid and thicker than the healthy leaves and small (Mead, 1998; Knapp et al., 1999). While the fruits becomes small and hard (Wirawan et al., 1998). CVPD disease is causing gram negative bacteria named Liberobacter (Sandrine et al, 1996).
Pathogens can not be cultured in vitro, but can be detected by PCR on 16S rDNA and electron microscopy (Hoy, 1998). Visual detection of CVPD disease has been difficult because the symptoms typically are chlorosis on the leaves that resemble symptoms of mineral deficiency, so it requires a special detection method. Detection of sensitive, rapid and accurate by PCR (Polymerase Chain Reaction), using a pair of specific primers O11 forward primer and reverse primer to amplify 16S rDNA O12c L. asiaticum that measures
ISSN ONLINE: 9 772303 337 008
approximately 1160 bp (Jagoueix et al., 1994).
The spread of the disease occurs primarily by insect vectors D. citri Kuwayama. The spread of the disease can also be caused by the spread of citrus plant seeds that have been infected by pathogens CVPD disease (tissue graft) (Capoor et al., 1974 in Mead, 1998). Preliminary research conducted by Wirawan and Furuichi (2003), in citrus plant that infected CVPD disease found a sugar-protein complex in the range of 100 kDa. Sugar-protein complex is believed to be the interaction of the protein produced by the virulent pathogen CVPD with plant protein which then affects the accumulation of sugar in the affected plants.
Analysis of protein C ends isolated and purified from the protein sugar complex showed 81% homology to the proteins that play a role in the transport of ions (Zn, Mn and Fe) in the plant cells, which raised the suspicion that infection inhibits ion transport in CVPD the citrus plant cells, causing symptoms of chlorosis and often described as a plant nutrient deficiencies. (Wirawan and Furuichi, 2003). Protein plays an important role in almost all biological processes, namely in catalysis (enzymes, hormones), transport, regulation of growth and differentiation. Analysis and purification of proteins can be done through three ways, namely electrophoresis, chromatography and ultracentrifugation. Amino acid sequence can be determined if the protein has been pure. By way of breaking the protein will be derived protein fragments specific to the amino acid sequence can be determined (Stryer, 2000).
The use of PCR using specific primer pair can detect infected plants CVPD more accurately. On citrus plant that infected CVPD disease likely to cause the interaction between plants and pathogens
CVPD, which can affect the protein found in plants. While the plants are healthy, there is not interaction between plants with the pathogen so that there will be only plant protein. Plant protein can be detected by using SDS-PAGE. Polyacrylamide gel can separate proteins by mass. Specific protein molecules of SDS-PAGE results will be known by comparing the protein bands citrus plants that infected and healthy.
Causes and vectors of CVPD disease
CVPD affected plants leaves undergo chlorosis, the symptoms resemble nitrogen deficiency, zinc, manganese and iron (Setiawan & Trisnawati, 1999). Outside the visible symptoms are as follows: a. Leaf yellowing or chlorosis, and color of the leaves become older bones. Increasingly pale leaves, the bone of leaves more clearly. b. The leaves become thicker and stiffer, usually smaller. c. Plant growth becomes stunted and young plants become stunted. Although the disease symptoms differ depending on the varieties of citrus, common symptoms are yellowing leaves on the bone, followed by yellowing of the entire leaf, suddenly the network clogging vessels (Su, 2001).

Fig. 1. Symptons of CVPD disease in citrus leaf (Wirawan et al, 2000)
CVPD disease-causing bacteria named Liberobacter. Liberobacter an Alpha Proteobacteria and have been successfully characterized by analysis of the 16S rDNA sequences and beta operon (rplkAJL -rpoBC) gene (Hocquellet et al., 1999). CVPD disease-causing pathogenic bacteria known to spread by insects such as fleas or also called citrus psyllid named D. citri Kuwayama. Insect D. citri as vector Liberobacter bacteria have the potential to breed high, especially in the lowlands and the period of transmission (infective period) can take quite a long time to 90 days. This insect can lay up to 800 eggs and the eggs may hatch after 3-5 days and a year later there were nine generations (Anonymous, 1996).
D. citri can become the most important vector in citrus if there are pathogens CVPD, but if there is no pathogen is usually considered a minor pest (Knapp et al., 1999). Damage caused by insects on plants grown citrus is orange leaves become wrinkled, curled or kinky, growth becomes stunted, besides the young leaves, imago also suck fluid stem cells of leaves, young shoots or other plant tissues young. D. citri can transmit the bacteria Liberobacter not only to citrus plants, but also to plants that are still related to the citrus and the citrus crops not like the periwinkle plant (Knapp et al., 1999).
Interactions between vectors and pathogens not yet known, the acquisition time is 30 minutes on D. citri (Asian psyllids) and 24 hours for the African psyllids. Pathogens proliferate in the vector's body. Adult insects fourth and fifth instar nymphs D. citri capable of transmitting pathogens after 8-12 days. Pathogens can be found in the haemolymph vector (Mead, 1998). There is a phenomenon of rapid deployment CVPD, although not found
insect vectors. Whiteside et al. (1988) suggest that vector does not seem efficient in the transmission and epidemics occur only if the population is high and there are many sources of inoculum. This raises the suspicion that the plant can contain bacteria from seed although it does not cause symptoms, which is reinforced by the presence of infected seedlings in land cultivators, even though it is stated that buds from trees that appear healthy and asymptomatic.
Polymerase Chain Reaction (PCR)
PCR analysis to detect CVPD disease performed using specific primers of 16S rDNA to detect the presence of pathogenic bacteria Liberobacter asiaticum on plants showing symptoms of disease. Isolation of DNA template made from the leaves of plants showing symptoms of disease in a manner as above. Primer sequences used are primer sequences specific to pathogens CVPD, namely O11 and O12. The primary use of this measure are amplified DNA is 1160 base pairs.
PCR analysis of the healthy citrus and infected CVPD using specific primers for CVPD result that the affected plants CVPD on the results of gel electrophoresis on 1% agarose contained the DNA bands at 1160 bp, whereas the healthy plants are not visible DNA bands (Figure 2). PCR analysis of the healthy citrus and infected CVPD shows that the citrus that infected were visible presence of DNA bands at 1160 bp, whereas in healthy citrus did not show any DNA bands at 1160 bp. This indicates that the affected plants contain bacteria Liberobacter asiaticum, because the presence of these bacteria was detected with primers which are conserved 16S rDNA sequences (sequences that must exist).
ISSN ONLINE: 9 772303 337 008
While on a healthy plant did not find any bacteria Liberobacter. According Jagoueix et al. (1994), detection of sensitive, rapid and accurate today is by PCR (Polymerase Chain Reaction) using specific primer pair of forward primer and reverse primer O11 O12c to amplify 16S rDNA Liberobacter asiaticum which measures approximately 1160 bp. Wirawan (2001), states that the cause of the disease CVPD in Bali is L. asiaticum.
M 1 2 3 4 5
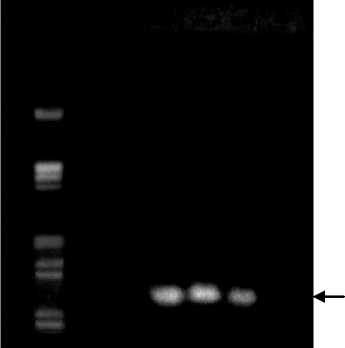
1160 bp
Fig. 2 Detection of pathogenic bacteria CVPD (Liberobacter asiaticum) of citrus plants
Protein analysis of Citrus Plants that infected CVPD disease by SDS-PAGE (Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis)
Electrophoresis is a standard method to separate, identify, characterize and purify molecules of DNA / RNA or protein. There are two kinds of gel that can be used in gel electrophoresis is polyacrylamide and agarose gels. They differ in the ability to separate different sizes of DNA (Toha, 2001). Citrus plant protein analysis was performed using polyacrylamide gel electrophoresis using as a separator. Polyacrylamide gel can separate proteins by mass. Proteins in the gel can be seen after stained with coomassie seen as a blue
streamers. Results of the analysis of protein for healthy tangerines and disease CVPD can be seen in Figure 3.
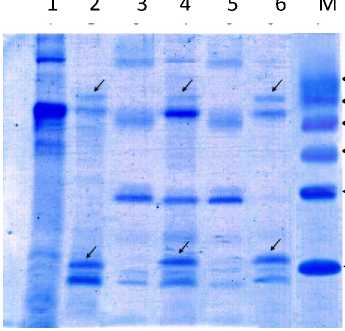
66 kDa
45 kDa
31 kDa
Fig. 3. Total protein in some types of citrus plant leaves. In citrus infected CVPD found specific proteins molecules of approximately 16 kDa and 66 kDa
Citrus plants that infected CVPD reveal any specific proteins molecules of approximately 16 kDa and 66 kDa, whereas the healthy plants did not reveal any of the specific protein. Protein molecule was detected in plants that infected CVPD disease can be a plant molecular reactions due to infection by pathogens and can be virulence proteins produced by pathogenic CVPD, or an interaction between the proteins of plant protein produced virulent pathogens. The existence of specific proteins thought to cause disruption transpot like Mn and Zn into the citrus plant cells, causing symptoms such plants Zn and Mn deficiency symptoms. This is in accordance with the opinion of Alberts et al. (1991), to be able to function properly, the protein that plays a role as a protein transpot will usually bind specific compounds (compounds of certain minerals such as Ca, Mg, Mn, Zn and others) to be able to move compounds pass through the membrane. The binding compound can be prevented in particular by a competitive barrier (competitive inhibitor)
to prevent binding between the compound dissolved (the solute) with protein, or a barrier is non-competitive (non-competitive inhibitor). Toha (2001), stating that a compound will transported must first bind to the protein-specific transpot so that the compound will be able to be taken across the membrane. Meanwhile, according to Sastrahidayat (tt), the enzyme or toxin produced by the bacteria may interfere with the metabolic processes of plants such as disorders of the ion transport into the plant cells.Transpot ion into plant cells occurs by several mechanisms which involve proteins in plant cell walls. The possible role of specific protein molecules that are detectable in the mechanisms of infectious diseases in citrus CVPD is as follows (Figure 4). The second interaction is detected specific protein (± 16 kDa and 66 kDa) are likely to clog the channel by binding (binding) domain of membrane proteins in the channel so that Zn and Mn can not enter into the plant cells. This is in accordance with Alberts et al. (1991), potein channel (channel protein) can only skip inorganic ions, especially Zn ++, Mn ++, Na +, K +, Ca ++ and Cl
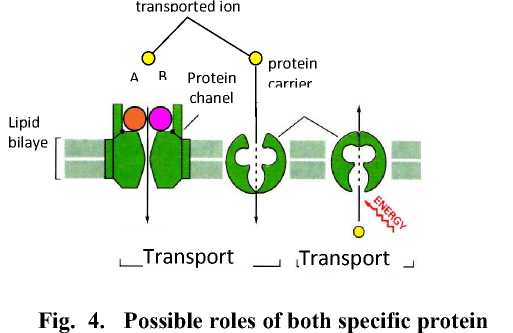
molecules with molecules of approximately 16 kDa and 66 kDa in mechanisms of infection
CVPD disease in citrus.A & B specific protein molecule
Analysis of proteins in citrus plants obtained results that citrus plant that infected CVPD disease found a specific protein with molecule of approximately 16 kDa and at 66 kDa, while the healthy citrus, there are no specific proteins. This suggests that there are differences in the formation of specific protein molecules in several types of citrus that infected pathogen. This may be caused by pathogens that infect plants are the same pathogens that Liberobacter asiaticum so that the plant protein interactions as a response mechanism of plant resistance to pathogens is the same protein. According to Callahan et al. (1997), the specific protein produced by the plant can be a reaction plant resistance to pathogens or protein is a protein produced by the pathogen or pathogens secrete incompatible elicitor released quickly, resulting in tissue damage as a hypersensitive reaction (HR). HR is a mechanism of plant resistance to pathogens and as an initial stage of a reaction between plant by plant pathogens usually involves some protein.
REFERENCES
Alberts, Bruce; Dennis Bray; Julian Lewis; Martin Raff; Keith Robert; James D. Watson. 1991. Molecular Biology of The Cell. Third Edition. Garland Publishing, Inc. New York & London.
Anonim. 1994. Pengelolaan Organisme
Pengganggu Tumbuhan Secara Terpadu pada Tanaman Jeruk.
Direktur Jenderal Pertanian
Tanaman Pangan Jakarta. 73 hal.
Callahan, FE; Johnie N. Jenkins; Roy G. Creech; Gary W. Lawrence. 1997. Integrated Pest Management Research Unit. Crop Science Research Laboratory, Missisipi State.
ISSN ONLINE: 9 772303 337 008
Feng, Teng-Yung. 1988. Molecular Understanding of Plant Pathogen Interaction. Institute of Botany. Academi Sincia.
http://botany.sinica.edu.tw/personnel /224.html
Hocquellet, A; Joseph M. Bove ; Monique Garnier, 1999. Isolation of DNA from Uncultured “Candidatus Liberobacter” Species Associated with Citrus Huanglongbing by RAPD. Curr Microbiol, 38:176 – 182. Springer Verlag New York, Inc.
Hoy, A. Marjorie. 1998. Citrus Psylla. Entomology and Nematology Department University of Florida, p.5.
http://extlab7.entnem.ufl.edu/PestAl ert.html
Jagoueix, S; J.M Bove and M Garnier. 1996. PCR Detection of The Two Candidatus Liberobacter Species Assosiated with Greening Disease of Citrus. Moleculer and Cellular Probes. 10: 43-50
Jagoueix, S; J.M Bove and M Garnier. 1997. Comparison of The 16S/23S Ribosomal Intergenic Region of Candidatus Liberobacter asiaticum and Candidatus Liberobacter africanum, The Two Species Assosiated with Citrus
Huanglongbing (greening) Disease. Int. J of Syst. Bacteriology. Jan. p 224-227.
Julyasih KSM., 2009. Perkembangan Bioteknologi Indonesia di
Universitas Udayana, Udayana University Press. 269 hal
Kato, Yasuhiro; Masao Sakaguchi; Yasuo Mori; Kumiko Saito; Tatsunosuke Nakamura; Evert P. Bakker; Yoko Sato; Shinobu Goshima; and Nobuyuki Uozumi. 2001. Evidence in Support of a Four Transmembranepore-Transmembrane Topology Model for the Arabidopsis thaliana Na+/K+ Translocating AtHKT1 Protein, a
Member of The Superfamily of K+ Transporters. Graduate School of Bioagricultural Science and
Bioscience Center, Nagoya
University, Japan.
Knapp, L. Joseph; Susan Halbert; Richard Lee; Marjorie Hoy; Richard Clark and Michael Kesinger. 1999. The Asian Citrus Psyllia and Citrus Greening Disease. Integrated Pest Management Florida..
Mead, F.W. 1998. Asiatic Citrus Psyllid Diaphorina Citri Kuwayama. University of Florida, Cooperative Extension Service. Institut of Food and Agricultural Services. http://creaturesifas.ufl.edu
Ohtsu, Y; M. Prommintara; S. Okuda; T. Goto; T. Kano; K. Nakashima, M. Koiszumi; J. Imada; and K. Kawashima. 2002. Partial
Purification of Thai Isolate of Citrus Huanglongbing (greening)
Bacterium and Antiserum
Production for Serological
Diagnosis. J. Plant Phatol. 68: 372377.
Sandrine, J., J.M Bove and Garnier. 1996. PCR Detection of The Two Candidatus Liberobacter Species Associated with Greening Disease of Citrus, Moleculer and Celluler probes, 10:43-50.
Sandrine, J., J.M Bove and Garnier. 1994. The Phloem Limited Bacterium of Greening Disease of Citrus is a Member of the a Subdivision of the Proteobacteria. Journal of
Systematic Bacteriology, 44:370-386.p.
Sastrahidayat, I.R. tt. Ilmu Penyakit Tumbuhan. Usaha Nasional.
Surabaya Indonesia.
Stryer, Lubert. 2000. Biochemistry. Edisi 4. Vol 1. Stanford University. Alih bahasa Tim Penerjemah Bagian Biokimia FKUI. Penerbit Buku Kedokteran.
Su, Hong-Ji. 2001. Departement of Plant Pathology and Entomology.
National Taiwan, University Taipei, Toha, A Abdul Hamid. 2001. Deoxyribo Nucleac Acid. Keanekaragaman, Ekspresi, Rekayasa, dan Efek Pemanfaatannya. Penerbit Alfa Beta Bandung. 111 hal.
Whiteside, J.O; S.M Garnsey and L.W Timmer. 1988. Compendium of Citrus Diseases. USA. APS Press
Wirawan, I.G.P. 1999. Metode Penelitian Bioteknologi. Beberapa Teknik dan Protokol. PS Pasca Sarjana (S2) Bioteknologi Pertanian. Universitas Udayana. 22 hal.
Wirawan, I.G.P; D.N Suprapta; N. Arya; M. Sudana; N. Widjaya; W. Susila. 2000. Karakteristik Patogen dan Isolasi Gen untuk Ketahanan Terhadap CVPD pada Tanaman Jeruk di Dati II Buleleng, Karangasem, Bangli, Gianyar dan Badung. Laporan Akhir
Penmelitian. Kerjasama Fakultas
Pertanian Universitas Udayana dengan Dinas Pertanian Tanaman Pangan Propinsi Daerah Tingkat I Bali. Denpasar.
Taiwan. R.OC.
Wirawan, I.G.P. 2001. Bioteknologi Menjawab Tantangan Pembangunan Berbasis Teknologi. Orasi Ilmiah Pengukuhan Guru Besar Tetap Universitas Udayana. 32 hal.
Wirawan, I.G.P dan Furuichi. 2003.
Detection of Sugar Protein Complex in Leaf of Citrus Infected by
Liberobacter asiaticum. Preliminary Research Report to JSPS. Nagoya University Bioscience Cente
ASIA OCEANIA BIOSCIENCES AND BIOTECHNOLOGY CONSORTIUM • 7
Discussion and feedback