THE WATER EXTRACT OF Saragassum fusiforme IS A POTENTIAL ELICITOR OF INDUCED RESISTANCE IN SOLANUM LYCOPERSICUM BUT NOT IN Nicotiana benthamiana
on
THE WATER EXTRACT OF Saragassum fusiforme IS A POTENTIAL ELICITOR OF INDUCED RESISTANCE IN SOLANUM LYCOPERSICUM
BUT NOT IN Nicotiana benthamiana
Layth Sbaihat, Daigo Takemoto and Kazuhito Kawakita*
Plant Pathology Laboratory, Graduate School of Bioagricultural Sciences, Nagoya University,
Chikusa--‐‑ku, Nagoya, 464--‐‑8601, Japan
*Corresponding author: kkawakit@agr.nagoya --‐‑u.ac.jp
ABSTRACT
Molecules called elicitors induce plants’ defense responses.Elicitor --‐‑induced resistance rarely leads to complete pathogen control, but reduce lesion size and/or number instead. We investigated a novel elicitor extractedfrom the brown sea algae Saragassum fusiforme), whichis sowed to induce reactive oxygen species production and protection against powdery mildew and late blight diseasesoftomato Solanum lycopersicum, cultivar Lady First)plants. On the other hand, the studied elicitor did not induce reactive oxygen species production in Nicotiana benthamiana plants nor protected them against late blight disease .
Keywords: Elicitor, Induced Resistance, Saragassum fusiforme, Solanum lycopersicum, Nicotiana benthamiana.
INTRODUCTIO N
lant pathogens are a major challenge
Pof plant growth and development.
They can seriously affect growth, production, and quality of agricultural crops. Plants exhibit various sorts of acquired resistance enabling them to overcome the invasion of the vast majority of potential pathogens Jones and Takemoto, 2004, Muthamilarasan and Prasad, 2013). The major trends of specialists to protect plants are disease control and developing resistant varieties. Nonetheless, improved resistance of pathogens to pesticides and fungicides, environmental and health concerns related to agro--‐‑chemical and long and costly breeding programs are considered major obstacles limiting the traditional plant protection methods Duvick, 2005, Lopes et al., 2012, De La Fuente, 2013, Nicholls and Altieri, 2013, Sierotzki and Scalliet, 2013). When interact with pathogens, plants activate defense reactions upon the recognition of small, structurally conserved motif molecules within many microbial species. These molecules are called microbe associated molecular patterns or pathogen associated molecular patterns MAMPs or PAMPs) Jones and Takemoto, 2004, Kouzai et al., 2013). Defense reactions lead to acquired resistance if they were sufficient and well--‐‑timed,
or can be overcome by the attacking pathogens.
Elicitors are agents that are able to induce plant’s resistance. The term ‘elicitor’ was originally used for compounds that induce accumulation of antimicrobial phytoalexins in plants,and is now commonly applied to agents stimulating any type of defense response Keen and Bruegger, 1977). An increasingly growing interest of scientists to explore new elicitors of plant resistance and understanding the molecular and biochemical basis of their action, is aiming at fostering plant protection strategies. Elicitors were extracted from bacteria, fungi, oomycetes, sea algae and plants or even chemically synthesized Nurnberger, 1999, Walters and Fountaine, 2009, Armana and Ul Qader, 2012).
Elicitor applications lead to resistance reactions including; the production of reactive oxygen species ROS), reactive nitrogen species RNS), production of phytoalexins, induction of hypersensitive reaction HR), callose deposition, lignin accumulation and expression of resistance related genes Doke and Tomiyama, 1980, Takemoto et al., 1999, Matsukawa et al., 2013, Monjil et al., 2013, Takeuchi et al., 2013). Elicitors can be species specific or nonspecific, and can induce resistance both locally and systemically. For example, pretreatment of oligogalacturonides produced by partial enzymatic degradation of the
cell wall of citrus fruit rinds on Ara bidopsis thaliana induced resistance reactions and the plants’ resistance against Botrytis cinerea Klarzynski et al., 2003, Suárez et al., 2013). The first chemical resistance activator, Probenazole, was registered in Japan as Oryzemate in 1975, and since then many other chemical and biological activators have been developed, including ASM, registered as Bion and Actigard Syngenta), Milsana Reynoutria sacalinensis extract; KHH BioScience), Elexa chitosan; SafeScience) and Messenger harpin protein; Plant Health Care) Walters et al., 2013). In this work, the eliciting ability of a crude water extract of the brown sea algae Saragassum fusiforme) was analysed for inducing ROS production in tomato Solanum lycopersicumcv. Lady First) and Nicotiana benthamiana plants. In addition, the ability of the extract to induce the resistance of S. lycopersicumandN. benthamiana against oomycete and fungal pathogens was also evaluated.
MATER IA LS AND METHODS
Biological materials and growth conditions: Tomato plants S.lycopersicum cv. Lady First) were germinated from seeds Aisan Seed C., Kiyosu, Japan) at 25--‐‑28 °C and then grown at 20--‐‑23 °C under a photoperiod of 16 h light/8 h dark period in environmentally controlled growth cabinets. Seeds of Nicotiana benthamiana were provided by the Leaf Tobacco Research Center Japan Tobacco Inc., Tokyo, Japan). N. benthamiana plants were grown at 23 °C and 70% humidity under a 16 h photoperiod and an 8 h dark period in environmentally controlled growth cabinets. The pathogenic isolate [Phytophthora infestans Mont.) De Bary], race 1.2.3.4 was used in the research; collection of zoosporangia and induction of zoospore production from P. infestans was performed. Zoosporangia suspensions from the P. infestans isolates were prepared as follows; P. infestans isolates were sub--‐‑cultured on rye--‐‑media for 7–10 days, 20 ml of water were added to the surface of the P. infestans colonies, which were then rubbed with a cotton swab to release the
zoosporangia, the zoosporangia suspension was then incubated at 10 °C for 3 hours for zoospores production. Tomato plants infected with the obligate biotroph Oidium spp. were provided by Aichi Agricultural Research Centre Aichi, Japan) and kept at 23--‐‑25 °C under a photoperiod of 16 h light/8 h dark period in environmentally controlled growth cabinets.
Inoculation: For plant--‐‑Pathogen
interaction tests; leaflets of S.lycopersicum and leaves of N. benthamiana plants were inoculated with 0.5 ml and 1 ml aliquots of P. infestans zoospore 105 zoospores/ml) suspension, respectively, and covered with lens papers. The inoculated plants were kept at high humidity at 20°C for 1 day, and moved then into 23 °C growth cabinet. S. lycopersicum plants were mixed with plants infected with Oidium spp, and the positions of the plants were changed randomly every two days to insure uniformity of exposure to the airborne pathogen. The inoculated leaves were observed on daily basis for monitoring disease symptoms.
Elicitor extraction and preparation: Brown sea algae S. fusiforme) were steamed at a temperature of 120 °C and a pressure of 2.0 kg/ cm2 for 60 minutes. The steam was trapped and cooled. The obtained solution was used as sea algal product AP).
Measurement of O2 --‐‑ production: To measure the relative intensity of O2--‐‑ generation,L--‐‑ 012--‐‑mediated chemiluminescence--‐‑based photon counting was developed. L--‐‑012 Wako, Osaka, Japan) is a luminol derivative that is highly sensitive to O --‐‑ Kobayashi et al., 2007). 2
To detect the O --‐‑production in S.lycopersicum
2
and N. benthamiana leaves, 0.5 mM L--‐‑012 in 10 mM MOPS--‐‑KOH pH 7.4) were infiltrated to the intercellular space through the abaxial surface of leaves using a syringe without a needle. Chemiluminescence was monitored continuously using a photon image processor equipped with a sensitive CCD camera in a dark chamber at 20 °C Aquacosmos 2.5; Hamamatsu Photonics, Shizuoka, Japan), and quantified using the U7501 program Hamamatsu Photonics).
R ESULTS AND DISCUSSIO N
Reactive Oxygen Species production activity
Signaling via reactive oxygen species ROS) is widely regarded to be central to disease resistance in plants Mehdy, 1994; Wojtaszek, 1997; Fobert and Despres, 2005; Torres et al., 2006). ROS is also involved in the pathogen--‐‑antagonist interaction in postharvest biocontrol systems Liu et al., 2013). ROS was reported to demonstrate direct antifungal and antimicrobial activity during pathogen infection In addition, ROS contribute to downstream biochemical pathways of resistance such as callose deposition, salicylic acid SA) signaling and the expression of pathogenesis--‐‑related PR) genes Alvarez et al., 1998 and Vellosillo et al., 2010). The superoxide anion O --‐‑) is one of ROS 2
that is activated in S.lycopersicum,N. benthamiana and other plants in response to pathogen infections and elicitor application Mehdy, 1994 andMatsukawa et al., 2013). In this work, we used O --‐‑ as a marker of induced resistance in
2
response to elicitor application. O --‐‑ producing
2
activity of S.lycopersicum and N. benthamiana
due to AP elicitor application was measured by using O --‐‑ unique luminous reagent L--‐‑012. Figure 2
1 shows noteworthy induction of O --‐‑ production
2
in leaves treated with AP elicitor at 1% and 10% concentrations measured 90 minutes after elicitors’ application. Figure 1 also shows that S.lycopersicum plants responded to algal elicitor applications in a dose respective manner. Quite
the reverse, the model solanaceous plant, N. benthamiana, did not show induction of ROS in response to algal elicitor treatments. Algal extract was applied to N. benthamiana leaves at several concentrations, and none of them showed superoxide accumulation Figure 4). Based on O --‐‑ 2
induction in S.lycopersicum and N. benthamiana, it might be claimed that algal product is a species specific elicitor; nevertheless, our results show
that the superoxide O --‐‑, which is produced in
2
ROS accumulation in addition to other resistance S.lycopersicum due to algal elicitor applications, reactions on potato plants as a result of algal is involved in the induced resistance against P. elicitor data not shown) which destabilizes this infestans.
hypothesis.
Protection against diseases
Induced resistance reactions of plants could lead to disease prevention or reduction. P. infestans is an economically important filamentous pathogen of potato. Moreover, it causes late blight disease on a range of solanaceous species including but not limited to tomato and N. benthamiana Becktell et al., 2006, Chaparro--‐‑
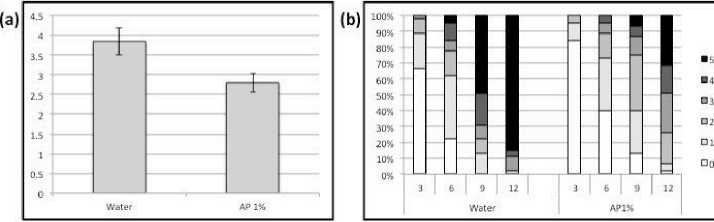
Garcia et al., 2011). We investigated the effects of preceding algal elicitor applications on late blight severity caused by P. infestans inoculated onto S.lycopersicum and N. benthamiana subsequently. Severity of late blight on S.lycopersicum plants was reduced by around 27% measured 12 dpi as a result of algal elicitor Figure 2--‐‑a). The numbers of infected leaves Figure 2--‐‑b) as well as disease severity Figure 2--‐‑b and c) were reduced on tomato leaves previously sprayed with algal elicitor. On the other hand, applications of algal elicitor on N. benthamiana did not result in reduced pathogen infection. Late blight symptoms were developed similarly on N. benthamiana elicitor--‐‑ treated and water--‐‑treated leaves Figure 5). The diminished severity of late blight on tomato leaves could be the results of two possible
factors; direct antifungal activity of the studied algal product, or induced resistance of tomato
which lead to reduced pathogenicity. Depending
on the fact that similar algal elicitor applications did not lead to reduced severity of late blight on N. benthamiana, we assume the first hypothesis is not valid. Moreover, algal elicitor showed no antifungal activity against P. infestans culture on
ray medium data not shown). On the second hand, algal elicitor induced the superoxide production and accumulation on treated S.lycopersicum leaves. O --‐‑ possessesantifunga
2
activity through it toxicity, and is also involved in other resistance reaction that might lead
to reduced disease development and partial plant protection. Together, our results indicate
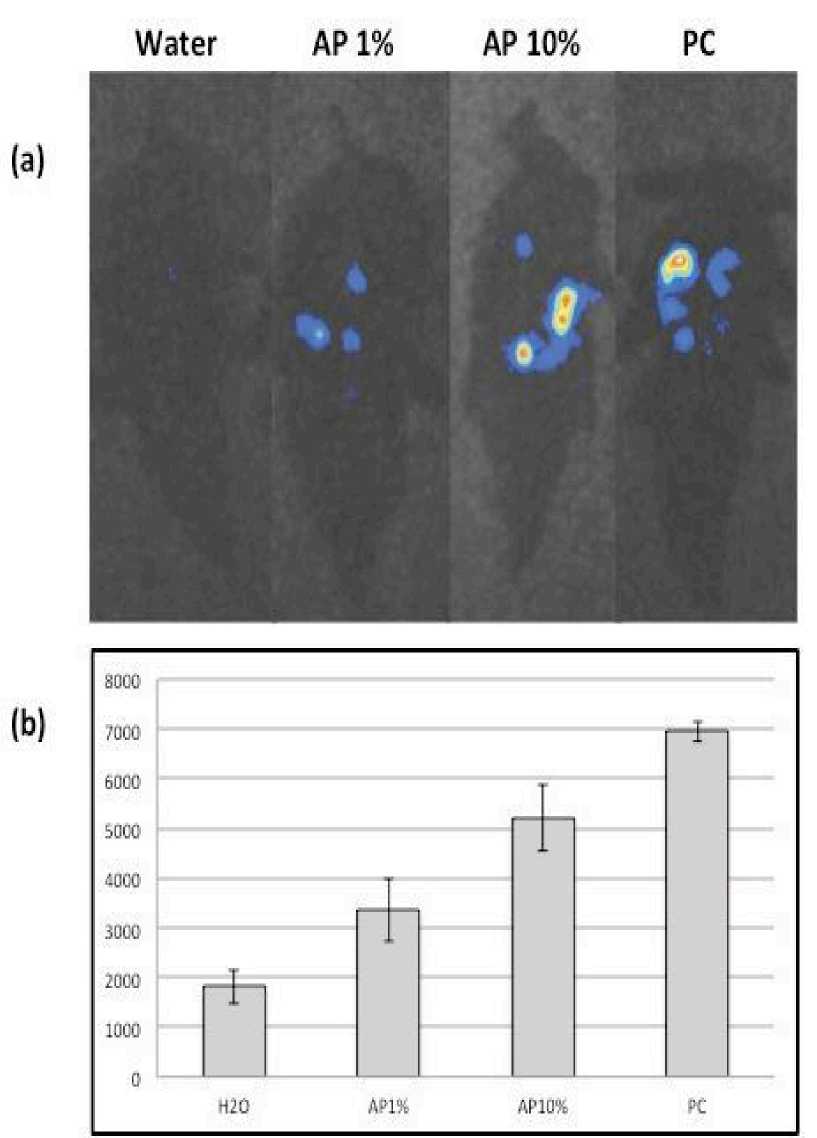
Fig. 1. Alga l Product AP) induced O2--‐‑ in tomato cv. Lady First LF) plants. a) S. lycopersicumcv. LF plants were sprayed with AP 1%, AP 10%, water as a negative control) or hyphal wall components of P. infestans as a positive control), and 90 min later the luminol derivative L--‐‑012 0.5 mM L--‐‑012 in 10 mM MOPS--‐‑KOH pH 7.4)) was infiltrated to the abaxial surface of the leaves using a needleless syringe . Chemiluminescence was monitored using a photon image processor equipped with a sensitive CCD camera in a dark chamber at 20 °C. Representative graphs were taken 90 mpt. b) Data were quantified using the U7501 program. Shown data are the a verage of three repeated experiments.
3 dpi
6 dpi
9 dpi
12 dpi
(c)
Water
AP 1%
Fig. 2. Pla nt--‐‑pathogen S. lycopersicumcv. Lady First--‐‑P. infestans) interaction in the presence of algal product AP) elicitor. Tomato cv. LF plants were thoroughly sprayed with AP 1%) or water and inoculated with 0.5 ml of P. infestans race 1.2.3.4) spores 106 spore/ml) 1 day later and kept in a humid chamber for another day, disease development was assessed daily from the 3rd until the 12th dpi. a) Disease severity on S. lycopersicum leaves 7 dpi, for disease severity quantification, the pathogens’ development on S. lycopersicum leaves was marked of 0 if the leaf is not infected) to 5 if the leaf is fully infected). b) Percentage of infected leaves and disease severity of the AP--‐‑ and water--‐‑treated plants from 3rd until 12th dpi.
c) Representative leaves of both treatments photographed on the representative days of the experiment. Three plants were used in each experiment. Results are the average of three repeated experiments.
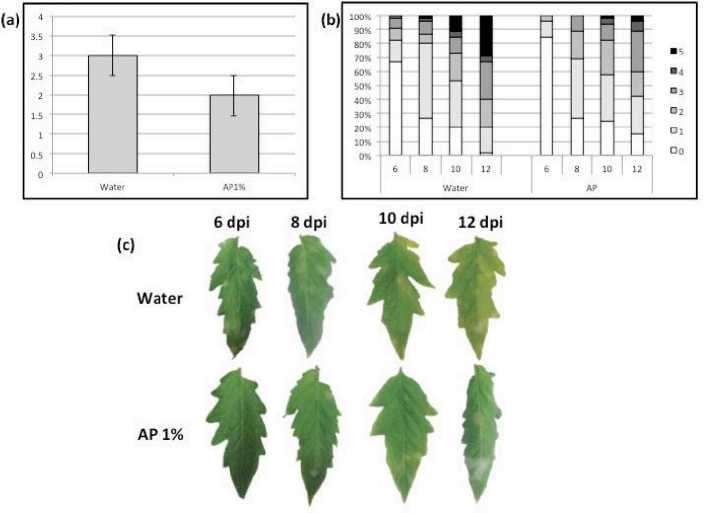
Fig. 3. Plant--‐‑pathogen S. lycopersicumcv. Lady First--‐‑Oidium spp.) interaction in the presence of algal product AP) elicitor. Tomato cv. LF plants were spray washed with AP 1%) or water and introduced into a growth chamber containing Oidium spp. infected plants 1 day later, disease development was assessed daily from the 6th until the 12th day after the introduction a) Disease severity on S. lycopersicum leaves 12 days after disease introduction, for disease severity quantification, the pathogens’ development on S. lycopersicum leaves was marked of 0 if the leaf is not infected) to 5 if the leaf is fully infected). b) Percentage of infected leaves and disease severity of the AP--‐‑ and water--‐‑treated plants from 6th until 12th day after pathogen introduction. c) Representative leaves of both treatments photographed on the representative days of the experiment. Three plants were used in each experiment. Results are the average of three repeated experiments.

Water
AP 100%
AP 1%
AP 50%
AP 10%
AP 25%
Fig. 4. Algal Product AP) did not induced O --‐‑2production in Nicot iana benthamina plants. a) N. benthamina plants were infiltrated through the abaxial leaf surface with AP 1%, AP 10%, AP 25%, AP 50%, AP 100%, water as a negative control) or hyphal wall components of P. infestans as a positive control), and 3 h later the luminol derivative L--‐‑012 0.5 mM L--‐‑012 in 10 mM MOPS--‐‑KOH pH 7.4)) was infiltrated to the abaxial surface of the leaves using a syringe without a needle . Chemiluminescence was monitored using a photon image processor equipped with a sensitive CCD camera in a dark chamber at 20 °C. Representative graph was taken 3 hpt.

Fig. 5. Plant--‐‑pathogen N. benthamina--‐‑P. infestans) interaction in the presence of algal product AP) elicitor. N. benthamina plants were thoroughly sprayed with AP 1%) or water and inoculated with 1 ml of P. infestans race 1.2.3.4) spores 106 spore/ml) 1 day later and kept in a humid chamber for another 24 h, disease development was assessed daily from the 2nd until the 12th day after inoculation. The experiment was repeated three times with similar results. Three plants were used in each experiment. Representative photographs were taken 7 dpi.
Following, we investigated whether algal elicitor prior applications lead to diminished--‐‑ severity of powdery mildew disease on tomato plants. Elicitor--‐‑treated S.lycopersicum plants were introduced into a growth chamber containing other plants infected with the air born pathogen Oidium spp. Figure 3 shows that the severity of powdery mildew on S.lycopersicum plant leaves was noticed less in the case of algal elicitor--‐‑treated plants compared to the control. Prior applications of algal elicitor have lead to reduced average disease severity on treated plants. The largest protection was noticed at the end of our tests i.e. 12 days after disease introduction Figure 3--‐‑a). Powdery mildew disease incidence and severity were delayed and reduced on algal elicitor treated plants Figure 3--‐‑b and c). Obviously, we have no means of investigating the direct effect of algal elicitor on the obligate biotroph Oidium spp.
Powdery mildew and late blight are considered among the major diseases threatening tomato culture around the world. Tomato powdery mildew caused by Oidium spp. is a relatively newly reported disease. Kashimoto et al. 2003) reported susceptibility of all commercial tomato cultivars available in Japan to powdery mildew caused by Oidium neolycopersici. All the cultivar showed highest level of susceptibility. Matsuda et al. 2005) revealed fungicide--‐‑tolerant isolates of tomato powdery mildew on naturally infected tomato leaves, indicating the necessity of alternative measures to control the pathogen. Late blight is a devastating disease of tomato as well. Once an unprotected tomato crop field, greenhouse, and/or plastic--‐‑cover cultures) is infected by P. infestans, the whole crop can be destroyed within 7 to 10 days Fry, 2008). Economic losses may be in the form of reduced yield, lower quality of the fruit, diminished storability and increased cost associated with fungicide applications Fontem et al., 1996). Alternative measures to control tomato powdery mildew and late blight seem vital. Induced resistance is an increasingly growing alternative method of plant protection. Our studied algal elicitor is able to induce resistance of tomato cv. Lady First plants leading to reduce powdery
mildew and late blight severities. However, it is obvious that algal product treatment did not completely suppress infection and disease development. Kuc 1982) reported that elicitor--‐‑ induced resistance rarely leads to complete pathogen control, but reduce lesion size and/ or number instead. However, reduced severity of the pathogen might lead to easier control. An elicitor added to certain fungicides, amplified the fungicides efficacy, which leaded to achieving the intended antifungal activity by applying highly reduced amount of fungicide Bounatesta et al., 2013). Purification of algal elicitor as well as combinations with other plant protectants might show better control of tomato powdery mildew and late blight diseases.
ACKNOWLEDGE MENTS
We thank the Leaf Tobacco Research Center 560 Japan Tobacco Inc., Tokyo, Japan) for N. benthamiana seeds, Ohta Oilmill Co.for providing the elicitor and Aichi Agricultural Research Center for providing Oidium spp.
REFERENCES
Armana N and Ul Qader SA. 2012. Structural analysis of kappa--‐‑carrageenan isolated from Hypnea musciformis red algae) and evaluation as an elicitor of plant defense mechanism. Carbohyd. Poly. 88: 1264--‐‑ 1271.
Aziz A, Poinssot B, Daire X, Adrian M, Bézier A, Lambert B, Joubert J, and Pugin A. 2003. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant--‐‑Microbe Interact. 16: 1118–1128.
Becktell MC, Smart CD, Haney CH, and Fry WE. 2006. Host–pathogen interactions between Phytophthora infestans and the solanaceous hosts Calibrachoa × hybridus, Petunia × hybrida, and Nicotiana benthamiana. Plant Dis. 90: 24--‐‑32.
Bounatesta R, Aubel GV and Cutsem PV; Auvelais BE), Marneffe BE) and Ottignies BE). 2013 Jul 18. Composition comprising
an elicitor of the plant immune system. United States Patent. US,2013,0302437A1.
Chaparro--‐‑Garcia A, Wilkinson RC, Gimenez--‐‑ Ibanez S, Findlay K, Coffey MD, Zipfel C, Rathjen JP, Kamoun S, and Schornack S. 2011. The receptor--‐‑like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana.” PLoS One 6: e16608. doi:10.1371/jour.
Cluzet S, Torregrosa C, Jacquet C, Lafitte C, Fournier J, Mercier L, Salamagne S, Briand X, Esquerré--‐‑Tugayé M--‐‑T and Dumas B. 2004. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Environ. 27: 917--‐‑928.
De La Fuente GN, Frei UK and Lübberstedt T. 2013. Accelerating plant breeding. Tre. Plant Sci.
Doke N and Tomiyama K. 1980. Effect of hyphal wall components from Phytophthora infestans on protoplasts of potato tuber tissues. Physiol. Plant Pathol. 16: 169--‐‑176.
Duvick DN 2005. The contribution of breeding to yield advances in maize Zea mays L.). In Advances in Agronomy Donald, L.S., ed.), Academic Press 83–145.
Fobert PR and Despres C. 2005. Redox control of systemic acquired resistance. Curr. Opi. Plant Biol. 8: 378–382.
Fontem DA, Nono--‐‑Womdim R, Opena RT, and Gumedzoe YD. 1996. Impact of blight infections to tomato yield. TVIS News. 1: 7--‐‑8.
Fry W. 2008. Review: Plant diseases that changed the world . Phytophthora infestans: the plant and R gene) destroyer. Mol. Plant Pathol. 9: 385–402.
Jones DA, and Takemoto D. 2004. Plant innate immunity--‐‑Direct and indirect recognition of general and specific pathogen--‐‑associated molecules. Curr. Opin. Immunol. 16: 48--‐‑62.
Kashimoto K, Matsuda Y, Matsutani K, Sameshima T, Kakutani K, Nonomura T, Okada K, Kusakari S, Nakata K, Takamatsu
S and Toyoda H. 2003. Morphological and molecular characterization for a Japanese isolate of tomato powdery mildew Oidium neolycopersici and its host range. J. Gen. Plant Pathol. 69: 176--‐‑185
Keen NT and Bruegger B. 1977. Phytoalexins and chemicals that elicit their production in plants. In ACS symposium series. 62: 1--‐‑26.
Klarzynski O, Descamps V, Plesse B, Yvin J, Kloareg B and Fritig B. 2003. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant--‐‑ Microbe Interact. 16: 115–122.
Klarzynski O, Plesse B, Joubert J, Yvin J, Kopp M, Kloareg B and Fritig B. 2000. Linear β--‐‑1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 124: 1027--‐‑1037.
Kobayashi A, Tai A, Kanzaki H and Kawazu K. 1993. Elicitor--‐‑active oligosaccharides from algal laminaran stimulate the production of antifungal compounds in alfalfa. Zeitschrift für Naturforschung 48: 575–579.
Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N and Yoshioka H. 2007. Calcium--‐‑dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19: 1065–1080.
Kouzai Y, Kaku H, Shibuya N, Minami E and Nishizawa Y. 2013. Expression of the chimeric receptor between the chitin elicitor receptor CEBiP and the receptor--‐‑like protein kinase Pi--‐‑d2 leads to enhanced responses to the chitin elicitor and disease resistance against Magnaporthe oryzae in rice. Plant Mol. Biol. 81: 287–295.
Kuc J. 1982. Induced immunity to plant disease. BioScience32: 854–860.
Liu J, Sui Y, Wisniewski M, Droby S and Liu Y. 2013. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Inter. J. Food Microbiol. 167: 153–160
Lopes MS, Reynolds MP, Manes Y, Singh RP, Crossa J. and Braun HJ. 2012. Genetic yield gains and changes in associated traits of
CIMMYT spring bread wheat in a ‘historic’ set representing 30 years of breeding. Crop Sci. 52: 1123–1131.
Matsuda Y, Mori Y, Sakano Y, Nishida M, Tarumoto K, Nonomura T, Nishimura H, Kusakari S and Toyoda H. 2005. Screening of wild Lycopersicon species for resistance to Japanese isolate of tomato powdery mildew Oidium neolycopersici. Breed. Sci. 55: 355--‐‑360.
Matsukawa M, Shibata Y, Ohtsu M, Mizutani A, Mori H, Wang P, Ojika M, Kawakita K and Takemoto D. 2013. Nicotiana benthamiana calreticulin 3a is required for the ethylene--‐‑ mediated production of phytoalexins and disease resistance against oomycete pathogen Phytophthora infestans. Mol. Plant--‐‑ Microbe Interact. 26: 880–892.
Mehdy MC. 1994. Active oxygen species in plant defense against pathogens. Plant Physiol. 105: 467–472.
Mercier L, Lafitte C, Borderies G, Briand X, Esquerré--‐‑Tugayé M and Fournier J. 2001. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 149: 43–51.
Monjil MS, Takemoto D, Shibata Y and Kawakita K. 2013. Bis--‐‑aryl methanone compound is a candidate of nitric oxide producing elicitor and induces resistance in Nicotiana benthamiana against Phytophthora infestans. Nitric Oxide 29: 34--‐‑45.
Muthamilarasan M and Prasad M. 2013. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 38: 1–17.
Nicholls CI and Altieri MA. 2013. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agron. Sustain. Dev. 33: 257–274.
Nurnberger T. 1999. Signal perception in plant pathogen defense, Cell. Molec. Life Sci. 55: 167–182.
Patier P, Potin P, Rochas C, Kloareg B, Yvin J and Linart Y. 1995. Free or silica--‐‑ bound oligokappa--‐‑carrageenans elicit laminarinase activity in Rubus cells and protoplasts. Plant Sci. 110: 27--‐‑35.
Sierotzki H and Scalliet G. 2013. A review of current knowledge of resistance aspects for the next--‐‑generation succinate
dehydrogenase inhibitor fungicides.
Phytopathology 103: 880--‐‑887.
Suárez L, Savatin DV, Salvi G, De Lorenzo G, Cervone F and Ferrari S. 2013. The non--‐‑traditional growth regulator pectimorf is an elicitor of defense responses and protects Arabidopsis against Botrytis cinerea . J. Plant Pathol. 95: 177--‐‑180.
Takemoto D, Maeda H, Yoshioka H, Doke N, and Kawakita K. 1999. Effect of cytochalasin D on defense responses of potato tuber discs treated with hyphal wall components of Phytophthora infestans. Plant Sci. 141: 219--‐‑ 226.
Takeuchi C, Nagatani K, and Sato Y. 2013. Chitosan and a fungal elicitor inhibit tracheary element differentiation and promote accumulation of stress lignin--‐‑like substance in Zinnia elegans xylogenic culture. J. Plant Res. 126: 811–821.
Thakur M. and Sohal BS. 2013. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. Article ID 762412.
Torres MA, Jones JDG, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141: 373–378.
Vellosillo T, Vicente J, Kulasekaran S, Hamberg M and Castresana C. 2010. Emerging complexity in reactive oxygen species production and signalling during the response of plants to pathogens. Plant Physiol. 154: 444–448.
Walters DR and Fountaine JM. 2009. Practical application of induced resistance to plant diseases: an appraisal of effectiveness under field conditions. J. Agri. Sci.147: 523–535.
Walters DR, Ratsep J and Havis ND. 2013. Controlling crop diseases using induced resistance: challenges for the future. J. Exper. Bot. 64: 1263–1280.
Wojtaszek P. 1997. Oxidative burst: an early plant response to pathogen infection. Biochem. J. 322: 681–692.
ASIA OCEANIA BIOSCIENCES AND BIOTECHNOLOGY CONSORTIUM • 19
Discussion and feedback