Phytochemical Content Identification And Antioxidant Activity Test Of Ethanol Extract Parijata Fruit (Medinilla Speciosa Blume) In Bedugul Area, Bali, Indonesia
on
INTERNATIONAL JOURNAL OF BIOSCIENCES AND BIOTECHNOLOGY eISSN: 2655-9994 pISSN: 2303-3371

Phytochemical Content Identification And Antioxidant Activity Test Of Ethanol Extract Parijata Fruit (Medinilla Speciosa Blume) In Bedugul Area Bali Indonesia
Gusti Ayu Willem Mintari1, I Ketut Suada1*, Trisna Agung Phabiola1, Nyoman Wijaya1, Ketut Ayu Yuliadhi1, Nor Kartini Abu Bakar2
-
1Agrotechnology Study Program, Facultyof Agriculture, Udayana University, Jl.P.B. Sudirman,Denpasar,Bali, 80235, Indonesia
-
2Department of Chemistry, Faculty of Science, Malaya University, Federal Territory of Kuala Lumpur, 50603, Malaysia
-
*Correspondence: ketutsuada@unud.ac.id
Received: 8-11-2023 Revised: 11-14-2023 Accepted: 12-29-2023
Citation: Mintari, G.A.W., Suada, I.K., Phabiola, T.A., Wijaya, N.,Yuliadhi, K. A.,Bakar, N., K. A.. (2023). Phytochemical Content Identification And Antioxidant Activity Test Of Ethanol Extract Parijata Fruit (Medinilla Speciosa Blume) In Bedugul Area, Bali. International Journal of Biosciences and Biotechnoogy, 11 (1): 24-30. https://doi.org/10.24843/IJBB.2023.v11.i01.p04
Abstract: Antioxidants are compound that function as free radical scavengers, these compounds can be found in parijata fruit (Medinilla speciosa Blume). The secondary metabolites of this fruit can be identified by the GC-MS method. The aims of study to identification of phytochemical content and test the antioxidant activity of the ethanol extract of Parijata fruit (Medinilla speciosa Blume) in the Bedugul area of Bali. This method is used because it is more sensitive and the results of the analysis are easy to understand. Using the GC-MS method, 11 organic compounds with a quality value of ≥ 90 were found in the ethanol extract of parijata fruit which have antioxidant, antimicrobial, and others. The 3 compounds with the highest Area Under Curve were 5-Hydroxymethylfurfural (AUC 31.05%); 1,2,3- Benzenetriol (AUC 22.32%); and 4H-pyran-4-one-2,3-dihydro-3,5-dihydroxy-6-methyl-(AUC 6.97%). These three compounds have antioxidant properties. Parijata fruit antioxidant activity test was tested with the DPPH method. The antioxidant activity of the ethanol extract of parijata fruit has an IC50 value of 11.26 mg/L, in the category of very strong antioxidant activity. So that with this category, parijata fruit can be given further treatment as food for health. In addition, compounds with antimicrobial properties can be used as biopesticide products in agriculture.
Keywords: Parijata Fruit, GC-MS, DPPH, Phytochemicals and Antioxidants
Central Laboratory for Genetic Resource and Molecular Biology
Faculty of Agriculture Udayana University https://ojs.unud.ac.id/index.php/jbb/index
Antioxidants are substances that are capable of providing endogenous protection and exogenous oxidative stress by capturing free radicals or molecules capable of inhibiting the oxidation of other molecules (Lai-Cheong and McGrath, 2017; Allemann and Baumann, 2008). Free radicals are molecules produced by the body's metabolism that can disrupt and negatively impact body activities. Free radicals that enter the body and attack healthy cells then accumulate. From this damage can cause premature aging, arteriosclerosis and cancer caused by tissue damage due to oxidation reactions (Miryanti et al., 2011). Several studies state that a number of plants have antioxidant activity (Mironczuk et al., 2018). One plant that has the potential to contain antioxidants is the parijata plant (Medinilla speciosa Blume). The parijata plant is a plant of the Medinilla clan, the Melastomataceae tribe. The parijata plant is beneficial for health when consumed according to needs, while the parts of the parijata plant that are useful include the parijata fruit which has a sour, bitter, and refreshing taste because the parijata fruit contains saponins, kardenolin, and flavonoids, while the leaves contain saponins, cardenolin, and tannins. (Zuhud et al., 2014). Parijata can also be used to help treat diarrhea and canker sores, and is used as an anti-inflammatory, anti-cancer and antibacterial ((Kementerian Negara Riset dan Teknologi Republik Indonesia, 2015). Parijata fruit is also called Asian grapes (Yugeswari et al., 2022) and has a pink and purplish-blue color, so according to Hasanah (2015), there is a type of antioxidant contained in red and blue fruits or foods. Based on the description above, it is necessary to study the identification of phytochemical content and test the antioxidant activity of the ethanol extract of Parijata fruit (Medinilla speciosa Blume) in the Bedugul area of Bali.
The equipments used in this study were autoclave, laminar air flow cabinet, vacuum rotary evaporator, gas chromatography-mass spectrophotometry, haemacytometer, microscope, blender, test tubes, petri dishes, erlemeyers, beaker glass, measuring cups, sprayers, micropipette, tweezers, scalpels, ose needles, cork borer, calipers, stationery, and camera. The materials used in this study were parijata fruits (M. speciosa Blume). Other materials used are methanol, DPPH, ethanol 96%), aluminum foils, tissue papers, and labels.
Parijata fruit samples were taken in Villa Enjung Bedugul Bali. The fruits then washed and clean with running water and chopped into smaller sizes. The samples were dried using an oven with 45oC temperature for 4x24 hours. The dried fuits then grinded with a blender to get simplisia. The simplisia then macerated with ethanol 96% for 3x24 hours and stirred periodically. The solution then filtered using filter paper until on the 2nd and 3rd screening. After the last filtering, all the filtrate collected and separated from all the dregs. The filtrate is concentrated using a rotary evaporator (RE), an ethanol extract was obtained and then weigh it.
Phytochemical content identification of ethanol extract parijata fruit compounds was carried out at the Bidlabfor Forensic Laboratory of the Bali Regional Police Departement. The extract was analyzed with Agilent 7890 MSD 5977B type Chromatography-Mass Spectrophotometry (GC-MS) Gas, with a Wakosil ODS/5C18-200 silica column with a size of 4.6 x 200 mm using nitrogen gas as a career. The injection temperature used was 290oC for 27 minutes with an injection rate of 1 ml/min.
Antioxidant activity test was carried out using the DPPH method. Ethanol extract of parijata fruit as much as 0,21647 g diluted in 5 ml of methanol as much as one thousand times then homogenized and centrifuged at 3000 rpm for 15 minutes and obtained extract solution concentration of 43,294 mg/L. Furthermore, the test solution is made 5 series concentrations of 0, 200, 300, 400, and 500 mg/L, then pipette 1 ml of the sample solution into each reagent bottle and add 1 ml DPPH solution with a volume ratio of 1: 1. Vortex the mixed solution and incubated in a dark and covered with aluminum foil with room temperature for 30 minutes. After that, each solution was measured absorbance using a UV-Vis spectrophotometer at that wavelength 517 nm. Then The percentage of antioxidant activity is calculated by the formula
. . . 4 . . AbsorbanceofBlank-AbsorbanceofDPPH
Antioxidants Activity = -------------------------------× 100%
Absorbance of Blank
The concentration value of each solution and the percentage calculation results Antioxidant activity was entered in the Microsoft Excel program get the linear regression equation curve of antioxidant activity y = ax + b, then converted to lC50 value. The y value is 50 and the x value is as concentration of the sample. Specifically, a compound is said to be very strong antioxidant if the IC50 value is less than 50 mg/L, strong for IC50 value of 50100 mg/L, moderate if IC50 value of 100-150 mg/L, and weak if IC50 is 151-200 mg/L (Mardawati et al., 2008; Molyneux, 2004).
The data obtained were qualitative data which is analyzed descriptively in tables and figures. The information obtained was then analyzed using PubChem and Microsoft Excel software programs. Then the compounds taken are compounds that have a quality value ≥ 90, compounds with these quality values indicate the possibility that the detected compounds have similar fragmentation patterns with the compounds in the provided library (Tamat, 2007; Sutar, 2013).
Parijata fruit (M. speciosa Blume) dried as much as 303 g was macerated with 96% ethanol solvent. The maceration results were weighed, 32.5 g of ethanol extract was gained, produced a rendement of 10.7%. The ethanol extract obtained then analyzed using GC-MS and tested its antioxidants activity.
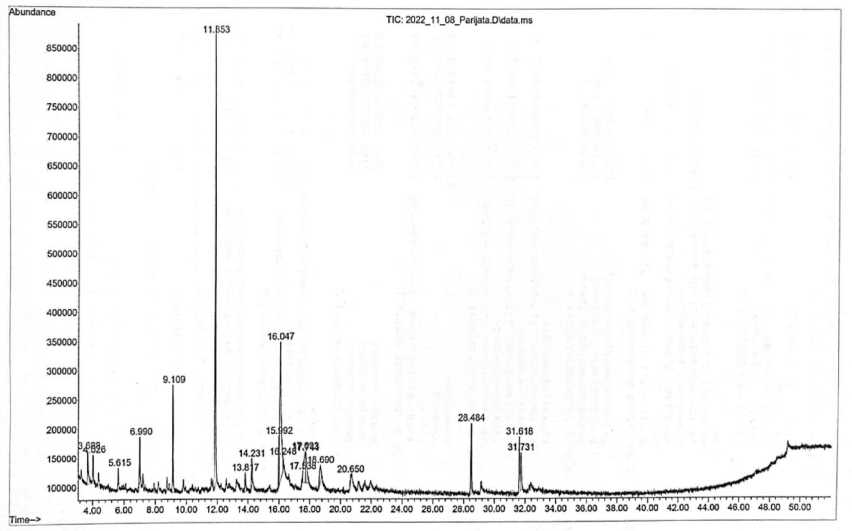
Figure 1. Chromatogram of parijata fruit ethanol extract
The results of the analysis of the content of compounds in the parijata fruit (M. speciosa Blume) using GC-MS showed that there were 11 compounds contained in the extract shown in Figure 1.
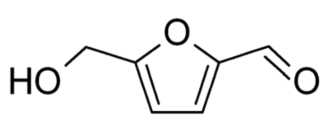
Figure 2. Compound structure of 5-hydroxymethylfurfural (Suhendar et al., 2019).

Figure 3. Compound structure of a 1,2,3 benzenetriol (Kartina et al., 2019).
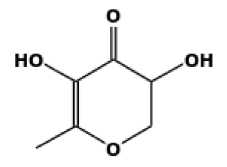
Table 1. Primary compunds found in extraxt ethanol of parijata fruit
|
No. |
Compund Name |
AUC (%) |
Rt |
|
1 |
5-hydroxymethylfurfural |
31.05 |
11.853 |
|
2 |
1,2,3-benzenetriol 4H-pyran-4-one-2,3- |
22.32 |
16.047 |
|
3 |
dihydro-3,5-dihydroxy-6-methyl- |
6,97 |
9.109 |
Figure 4. Compound structure of a 4H-pyran-4-one-2,3-dihydro-3,5 dihydroxy-6-methyl- (Wiradnyani dan Puspaningrum, 2019).
The main component contained in extraxt ethanol of parijata fruit (M. speciosa Blume) was 5-hydroxymethylfurfural with an AUC value of 31.05% at a retention time of 11.853, this compound has an antioxidants, fine chemicals, pharmaceutical ingredients, solvents, resins, and antifungal property (Hastuti, 2012; Utami et al., 2017; Kezia et al., 2021). 1,2,3-benzenetriol is the second most constituent compounds found in the extract with an AUC ratio of 22.32% at a retention time of 16.047. This compounds function as antioxidant (Ghopalakhrishnan, 2011). The third highest content are 4H-pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- at a time of 6.97 with an AUC value of 9.109%. This compounds function as antioxidant, antimicrobial, antiinflammatory, fragrance as a mixture of perfumes and cosmetics (Florentina et al., 2006; Ehsan et al., 2011; Meng et al., 2021). Successively, these compounds structure is shown in figures below.
Antioxidant Activity Test of Parijata Fruit Ethanol Extract. Parijata fruit ethanol extract antioxidant activity test is conducted by the DPPH method. DPPH is a free radical molecule with a purple color that could change into the yellow color stable compound with antioxidant reaction.
Table 2. The result of antioxidant activity measurements using UV-Vis spectrophotometre
Extract Absorbance Antioxidant (%)
concentrate
(mg/L
|
0 |
0,5866 |
0 |
|
8,6588 |
0,3366 |
42,6184 |
|
12,9882 |
0,2341 |
60,0920 |
|
17,3176 |
0,1361 |
76,7985 |
|
21,6470 |
0,0687 |
88,2884 |
The correlation between the concentration of the extract and the antioxidant activity of the ethanol extract of parijata fruit obtained by the equation y = 4.1251x + 3.5537. The regression equation is used to gain the IC50 value. By determining the value of y = 50 so that the IC50 is 11.26 mg/L and it is included in the very strong category, according to Mardawati et al. (2008) and Molyneux (2004), because the smaller the IC50 value, the more active a sample being tested to become an antioxidant compound.
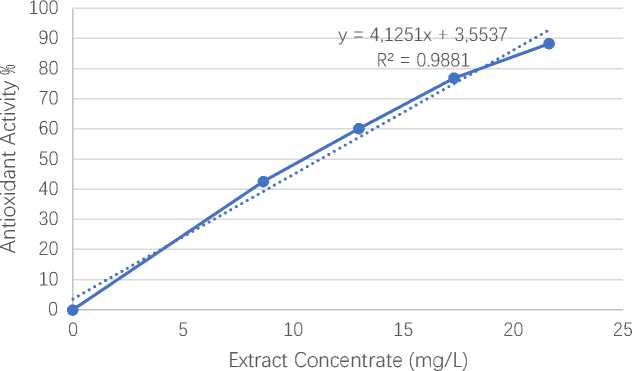
Figure 5. Linear regression curve of antioxidant activity
Compounds contained in the fruit extract of M. speciosa were, 2-Furancarboxaldehyde, 5-methyl-; Benzyl alcohol; 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl-; 5-Hydroxy-methylfur-fural; 1,2,3-Benzene-triol; Hexadecanoic acid-methyl ester; 9,12-Octadecadienoic acid (Z,Z)-, methyl ester; 9,12-Octadecadienoic acid-methyl ester; 9,12,15-Octadecatrienoic acid methyl ester, (Z,Z,Z)-; n-Propyl 9,12,15-Octadecatrienoate; dan 9,12,15-Octadecatrienoic acid, (Z,Z,Z)-. The main compounds contained in the extraxt ethanol of parijata fruit (M.
speciosa Blume) are presented in Table 1.
This method is used as it is simple, easy, fast and sensitive and only requires a small number of samples to evaluate the antioxidant activity of natural compounds, so it is widely used to test the ability of compounds to act as electron donors (Molyneux, 2004; Sanchez-Moreno, 2002). Parijata fruit ethanol extract created through the lowest to the highest concentration of DPPH solvent and being measured its absorbance absorption UV-Vis spectrophotometry using 517 nm wavelength. The results show as in Table 2.
According to table 2, each concentration has a different absorbance value and percentage of antioxidant activity. The higher the concentration of the extract, the absorbance value decreases but the percentage of antioxidant activity increases. A decrease in the absorbance value indicates that the DPPH molecule has been successfully reduced due to a reaction with an antioxidant compound (Molyneux, 2004). It was shown that the largest extract concentration, namely 21.6470 mg/L, had an absorbance value of 0.0687 and an antioxidant activity percentage of 88.2884%. Then, data in Table 2 shows the curve of correlation equation by parijata fruit ethanol extract with % antioxidant activity. As for curve linear regression equation from antioxidant activity test of parijata fruit ethanol extract is shown in figure 5.
Parijata fruit ethanol extract has very strong antioxidant activity because it contains secondary metabolites such as 5-Hydroxymethylfurfural; 1,2,3-Benzenetriol; and 4H-pyran-4-one-2,3-dihydro-3,5-dihydroxy-6-methyl-which is known to act as an antioxidant and belongs to the flavonoid and phenol class. According to Hardiana et al. (2012), the flavonoid class compounds act as antioxidant activity because they have hydroxyl class that can donate their hydrogen atoms to free radical compounds. Phenol is the main compound of phenolic which is widely found in plants. The higher the total phenol in a feed ingredient means it will show high antioxidant activity (Sandrasari, 2008).
The ethanol extract of parijata fruit (Medinilla speciosa Blume) contains 11 organic compounds. The compound with the highest percentage content was 5-hydroxymethylfurfural with an AUC value of 31.05%, followed by 1,2,3-benzenetriol with an AUC value of 22.32%, and 4H-pyran-4-one-2,3-dihydro-3 ,5-dihydroxy-6-methyl- with an AUC of 6.97%. These three compounds have uses as antioxidants. And also, the antioxidant activity of the ethanol extract of parijata fruit (M. speciosa Blume) has an IC50 value of 11.26 mg/L in the very strong category.
Author Contributions
Conceptualization, G.A.W.M and I.K.S.; methodology, G.A.W.M and I.K.S; software, G.A.W.M and I.K.S; validation, G.A.W.M and I.K.S.; formal analysis, G.A.W.M and I.K.S; investigation, G.A.W.M and I.K.S; resources, G.A.W.M and I.K.S; data curation, G.A.W.M and I.K.S; writing—original draft preparation, G.A.W.M and I.K.S; writing—review and editing, G.A.W.M and I.K.S; visualization, G.A.W.M and I.K.S; supervision, G.A.W.M and I.K.S; project administration, G.A.W.M and I.K.S.; funding acquisition, G.A.W.M and I.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable
Informed Consent Statement
Not applicable
Data Availability
Not applicable
Acknowledgements
—
Conflicts of Interest
The authors declare no conflict of interest
References
Allemann, I. B., Baumann, L. (2008). Antioxidants used in skin care formulations, 1–8.
Ehsan, O., Norhani, A., Syahida, A., Wan, Z. S., Abdul, R.O., Yin, W.H. (2011). Bioactive compounds and
biological activities of Jatropha curcas L. Kernel meal extract. International journal of molecular science 12:5955-5970.
Florentina., Mulyati., Tahan., Himma. (2006). Pemanfaatan tumbuhan sebagai bahan obat. Pusat penelitian biologi. Bogor: 333-339.
Ghopalakrishnan, S. (2011). GC-MS analysis of some bioactive constituents of Mussaenda frondosa Linn. Intl. J. Pharma. and Bio. Sci., 2(1):313-320.
Hardiana, R., Rudiyansyah, T.A.Z. (2012). Aktivitas antioksidan senyawa golongan fenol dari beberapa jenis tumbuhan famili Malvaceae. Jurnal Kimia Khatulistiwa, 1(1): 8–13.
Hasanah, N. (2015). Aktivitas antioksidan ekstrak etanol daun salam. Pena Medika Jurnal Kesehatan, 5(1).
Hastuti, N.D. (2012). Pembuatan Minuman Fungsional dari Madu dan Ekstrak Rosella (Hibiscus sabdariffa Linn.).
Teknologi Pangan: Media Informasi dan Komunikasi Ilmiah Teknologi Pertanian, 3(1): 29-63.
Kartina., Agung, M.W., Adiwena, M. (2019). Karakterisasi kandungan fitokimia estrak daun karamunting (Melastoma malabatchricum L.) menggunakan metode gas chromatography mass spectrometry (GC-MS). Biota: Jurnal Ilmiah Ilmu-Ilmu Hayati 16-23.
Kementerian Negara Riset dan Teknologi. (2015). Medinilla speciosa. http://www.warintek.ristek.go.id.
Kezia, K., Khalimi, K., Phabiola, T.A. (2021). Identifikasi senyawa antijamur dari agens hayati rizoplan. Jurnal Agroekoteknologi Tropika, 596-605.
Lai-Cheong, J E., McGrath, J.A. (2017). Structure and function of skin, hair and nails. Medicine (United Kingdom) 45(6):347–351.
Mardawati, E., Filianty, F., Harta, H. (2008). Kajian aktivitas antioksidan ekstrak kulit manggis (Garcinia mangostana L.) dalam rangka pemanfaatan limbah kulit manggis di kecamatan Puspahiang kabupaten Tasikmalaya.
Meng, F.H., Wang, X.W.A.J.Y., Wei, C. (2021). Study on the effect of Jajabe Powder on the quality of bread. Journal of food technology & nutririon sciences (3):121.
Mironczuk., Chodakowska, I., Maria, A., Witkowska, M.E., Zbieta, Z. (2018). Endogenous non-enzymatic antioxidantsin the human body Iwona. Adv Med Sci J, 63(1):68–78
Miryanti, Y.A., Sapei, L., Budiono, K., Indra, S. (2011). Ekstraksi antioksidan dari kulit buah manggis (Garcinia mangostana L.). Laporan penelitian lembaga penelitian dan pengabdian masyarakat. bandung: universitas katolik parahyangan
Molyneux, P. (2004). The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity, Journal Science of Technology.
Sanchez-Moreno, C. (2002). Review: Methods used to evaluate the free radical scavenging activity in food and biological systems, Food Sci. Technol. Int., 8(3):121-137.
Sandrasari, D.A. (2008). Kapasitas antioksidan dan hubungan nilai total fenol ekstrak sayuran Indigenous. Sekolah Pascasarjana. Institut Pertanian Bogor. (Tesis).
Suhendar, U., Fathurrahmanb, M., Sogandi. (2019). Antibacterial activity and mechanism of actionof methanol extractfrom kasturi mango fruit (Mangifera casturi) on caries-causing bacterium Streptococcus mutans. Jurnal kimia sains dan aplikasi 22(6):235-241
Sutar., Reza, M., Eka B.P., Zulianti, F., Annajiah, W., Fadhila, M., Avivah, P.N., Nurhafiza., Rahmawati, M., Halawiyah, A., Doni, S., Permana, R.S., Nurhayanti., Rais, L.B., Fauzana, D., Kholisoh, G. (2013). Laporan praktikum analisis instrumen GC-MS. Universitas Islam Negri. Syarif Hidayatullah Jakarta.
Tamat, S.R., Wikanta, T., Maulina, L.S. (2007). Aktivitas Antioksidan dan Toksisitas Senyawa Bioaktif dari Ekstrak Rumput Laut Hijau Ulva reticulata Forsskal. Jurnal Ilmu Kelautan Indonesia. 5:31-36.
Utami, S.P., Amin, N.A.S. 2017. Pembuatan 5-Hydroxymethylfurfural dari Glukosa melalui proses hot compressed water dengan variasi waktu dan suhu. Jurnal sains dan teknologi 16(2): 54-61.
Wiradnyani, N.K., Puspaningrum, D. (2019). Senyawa penyusun hasil fraksi etil asetat minuman sinom campuran jeruk nipis dan madu (Curcuma domestica val-tamarindus indica l.).
Yugeswari, V., Pebryani, N.D., Paramita, N.P.D.P. (2022). Penyandra kalistuayuan: the blessing of parijoto. BHUMIDEVI: Journal of Fashion Design 2(1):138-147.
Zuhud, E.A.M., Sinroyo., Sandra, E., Hikmat, A., Adhiyanto, E. (2014). Buku Acuan Umum Tumbuhan Obat Indonesia jilid 6. Dian Rakyat, Jakarta.
30
Discussion and feedback