Morphological and Chemical Characteristics of Porang Tubers (Amorphophallus oncophyllus) From Different Harvest Periods
on
Morphological and Chemical Characteristics of Porang Tubers (Amorphophallus oncophyllus) From
Different Harvest Periods
Gusti Setiavani, G., Budi Suarti, B., Mulia, M.N., Novita, A. & Gandaseca, S.
MORPHOLOGICAL AND CHEMICAL CHARACTERISTICS OF PORANG TUBERS (Amorphophallus oncophyllus) FROM DIFFERENT HARVEST PERIODS
Gusti Setiavani1*, Budi Suarti2, Mona Nur Moulia3, Aisar Novita2, Seca Gandaseca4
-
1Medan Polytechnic of Agricultural Development, Jalan Binjai KM. 10, Medan, 20002 Indonesia
-
2Faculty of Agriculture, Universitas Muhammadiyah Sumatera Utara, Jl. Muhtar Basri No.3, Kecamatan Medan Timur, Kota Medan, Sumatera Utara, 20238, Indonesia
-
3Politeknik Enjiniring Pertanian Indonesia, Jl. Sinarmas Boulevard Situ Gadung, Tangerang, Banten, 15339, Indonesia
-
4Department of Forestry Science, Faculty of Forestry, Universiti Putra Malaysia, UPM Serdang, Selangor, 43400, Malaysia
*Corresponding author: gustisetiavani80@gmail.com
ABSTRACT
Received:
11 March 2023
Accepted:
16 July 2022
Published:
31 July 2023
Porang tubers (Amorphophallus oncophyllus) have recently garnered more attention with the increasing demand for their derivative products. This research aimed to determine the morphological and chemical characteristics of porang tubers from different harvest periods. The method used was a completely randomized design. Also, for comparison, the morphological and chemical characteristics of bulbils were obtained from previous studies. Based on the analysis, morphologically, the stem tubers have an oval shape developing at the base of the stem with yellowish-brown to orangish-brown skin. Bulbils have an irregular oval shape with brown skin and white spots. They all have similarly dark yellow fibers. The stem tubers in the first harvest period were smaller in diameter and lighter (479.20 ± 183.54 g) than in the second period (609.71 ± 169.42 g). In contrast, bulbils at the leaf axils are smaller (diameter = 3–3.7 cm, thickness = 1.9– 2.4 cm) and only weigh about 12.3–25.3 g. Chemical analysis revealed that the flour made from the stem tubers contained 14.28–17.57% glucomannan and 9.16–11.10% protein, generally higher than bulbils with 25.78% glucomannan and 9.52% protein (very low). The yields of porang flour were 0.15±0.02% and 0.14±0.01% from the first and second harvest.
Keywords: bulbil, glucomannan, porang flour, porang tuber, yield
INTRODUCTION
Porang (Amorphophallus oncophyllus), also known as iles-iles in Indonesia, is a tuberous herbaceous shrub generally found in forests, under clumps of bamboo, and on mountain slopes (Sitompul et al., 2018). It grows more commonly in shaded areas with 40–60% light intensity (Yuniwati et al., 2020). Not long ago, this plant was less popular than true bulbs like garlics or tubers like potatoes. Considered nuisance weeds that offer no benefit, it grows wild or naturally without being cultivated or cared for. However, in recent years, the export demand for porang tubers in the form of dried slices or chips has grown dramatically, increasing the public’s interest in cultivating it as a cash crop.
In North Sumatra, porang has been cultivated since 2019 in many plantation areas, totaling about 621 ha. The government has implemented numerous strategic programs to support its cultivation and enhance its use value. Minister of State Owned Enterprises assigned Perhutani, a state-owned company responsible for forest resource management, to promote and manage porang development programs in industrial forests.
This plant can only grow one tuber per stem or called a single tuber (Saleh et al., 2015). The tuber consists of stem tubers that are located near the soil surface and bulbils that grow at the leaf axil or the base of the stem or leaf stalk. Stem tubers are the most widely used part. They are round and large with dull yellow to yellowish-brown flesh (Sari and Suhartati, 2015).
The main component of stem tubers is glucomannan, which is a type of carbohydrate. Dried stem tubers contain 15–64% glucomannan (Faridah et al., 2012). At this range, they have enormous potential as food ingredients
and staple food, especially to support national food security. Glucomannan is water soluble and can be fermented (Purwanto, 2014). Other distinctive properties include the ability to form a thick solution, expand, and gelatinize in water, form an impermeable layer (with the addition of NaOH or glycerin), and melt like agar (for which, it is used as microbial growth media) (Koswara, 2013).
Porang tubers are often mistaken for elephant foot yam (suweg in Indonesian). Even though they belong to the same family Araceae, their plant structures and tubers are different. Porang tubers are generally harvested three times when the plant dries up, with each growing cycle lasting about 7–12 months. At this time, the stem tuber has a higher glucomannan content than when harvested before the plant falls over (Chairiyah et al., 2014).
METHODOLOGY
Materials and Equipment
The main research material was Porang tubers, harvested from Amorphophallus oncophyllus plants cultivated by farmers in Deli Serdang, North Sumatra. Other materials included bottled drinking water from PT. Aqua, coarse salt, and filter papers. The equipment was a tuber slicer, disc mill, sieve, digital electronic balance (BL-2200H, Shimadzu, Kyoto, Japan), blender, drying oven (Terada Seisakusho, ED-4K-SP, Shizuoka, Japan), desiccator, shaker (Heidolph), spectrophotometer (Medilab), cuvette, vortex (LW Scientific Inc.), centrifuge (Universal Model: PLC-012E), LH magnetic overhead stirrer (VELP Scientifica Srl), caliper, and laboratory glassware.
Morphological Analysis
Ten samples of porang tubers collected from the first and second harvest periods were visually observed to sort out defective or rotten ones. Healthy tubers were characterized morphologically, including size (thickness and diameter), shape, color (flesh and fiber), and weight. The size was measured using a caliper, while the other attributes were observed visually (Minantyorini and Somantri, 2002).
Sample Preparation
Stem tuber samples were prepared into dried slices and then ground into flour. First, fresh tubers were peeled with a knife, washed, and cut thinly (2 mm) with a tuber slicer. Then, 1.5 kg of the sliced tubers were washed with 4.5 L of drinking water from PT. Aqua and left to dry. The tuber slices were immersed in a 5% w/w salt solution made by dissolving 50 g of salt (PT. Brataco Chemica, Bogor) in 1 L of water for 1 h and then thoroughly rinsed to remove any remaining salt. After sun drying for about 4–6 h, the dried and brittle tuber slices (called chips) were weighed, and the yield was analyzed to determine the relationship between harvest period and yield.
To analyze the moisture and glucomannan contents, the dried slices were ground using a blender. The resulting flour was weighed, passed through a 100-mesh sieve, and stored at 5 °C for further analysis. Observations were made in triplicates.
Chemical Content Analysis
The chemical analysis was conducted to examine the moisture, protein, and glucomannan contents of porang tubers. The moisture content was determined with the oven-drying
method (AOAC, 2012), protein with the Kjeldahl method (AOAC, 2012), and glucomannan with the gravimetric method (Widjanarko and Johana, 2015). To quantify the glucomannan content, the porang flour and aluminum sulfate (10% of the flour’s weight) were dissolved in warm water at 75 °C in a ratio of 1:10 (w/v) and continuously stirred for 35 min. The precipitate formed was separated using a centrifuge at 2000 rpm for 30 min. Then, the supernatant was collected and added with isopropyl alcohol at a 1:1 ratio (v/v) while continuously stirred until clumps were formed. Clumps were filtered through a filter paper, dried at 60°C for 24 h, and then weighed. The glucomannan content was calculated using the eq. 1.
Glucomannan (%) = t c^ry w<;l,ght . xW0 %(1)
Initial sample weight ' 7
Yield
The yield was calculated from the weights of tuber flour and slice (Muchtadi et al., 2013) using the eq. 2.
Weiqht of Poranq Flour (q)
Yield (%) = ---∏ × 100%(2)
Weight of Porang Slices (g)
Statistical analysis
This research employed a completely randomized design with different harvest periods as the treatments. Therefore, statistical analysis was conducted to determine if different harvest periods resulted in statistically different morphological and chemical properties. It consisted of ANOVA, least significant difference (LSD). The computer programs used were Microsoft Excel, SPSS v.20, and SMARTSTATXL. A Pearson
correlation test was performed to
determine the correlation between yield and harvest period.
RESULTS AND DISCUSSION
Morphological Characteristics of Porang Tubers
Porang tubers were obtained from plantations in Deli Serdang Regency, North Sumatra. As seen in Figure 1, stem tubers have a symmetrical oval shape with a basin in the middle, fibrous roots, and yellowish-brown to orangish-brown skin. These characteristics
correspond to Shaleh, et al, (2015). In comparison, bulbils have an irregular oval shape with brown skin and white spots.

Figure 1. Visual appearances of porang tubers: stem tubers (a) and bulbils (b).
Stem tubers have dark yellow flesh with yellow fibers. However, it was found that the yellow fibers from the first harvest were brighter than the second harvest (Figure 2), and some tubers were observed to have reddish-yellow fibers. Purwanto (2014) stated that porang tubers are sometimes called yellow porang (locally, iles kuning) because they have bright yellow flesh with fine fibers. Age at harvest affects tuber’s brightness (L*) and color (b*) (Irawan et. al., 2017). Among starchy corms, Xanthosoma sagittifolium has the highest L* value and the lowest browning index, meaning it is the brightest and most white. The whiteness of tuber flesh is strongly influenced by the polyphenol content that is responsible for enzymatic browning due
to a reaction with oxygen in the air (Lintang et al., 2017).

Figure 2. Colors of the skin, flesh, and fiber of porang tubers harvested in the first period (a) and the second period (b).
Statistical analysis showed that the diameters of porang tubers in the first and second harvest periods were significantly different (p < 0.05). After harvest, the tuber’s weight and water content can decrease due to respiration and transpiration (Gusmalawati et al., 2021). Tuber weight is correlated with glucomannan content. Glucomannan is responsible for 11% of variations in tuber weight, while the remaining 89% are influenced by other factors (Chairiyah et al., 2014). In addition to harvest time, environmental conditions strongly affect crop productivity (Ubi and Lam, 2020).
Yield from Different Harvest Periods
The yield was measured for the amount of flour produced from each unit of weight of dried slices (chips). Statistical analysis found no significant difference in yield from the tubers harvested in the first and second growing periods (p > 0.05). The average yields were 0.15 ± 0.02% for the first harvest and 0.14 ± 0.01% for the second harvest. Variations in the flour yield can be linked to the time of harvest (Lintang, et al., 2017) and genetic differences like variety, place of growth, chemical
composition, and water content (Irawan et al., 2017).
Water, Protein, and Glucomannan Contents
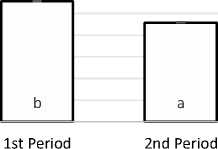
In addition to yield, this research also determined the chemical characteristics of porang flour, including water, protein, and glucomannan levels. The average water content of the flour was about 5 %. Statistical analysis showed no significant difference between the water contents of flour made from porang tubers in the first and second harvest periods (p > 0.05). In contrast, the protein contents of the flour from the two harvest periods were significantly different, as seen in Figure 3. The first harvest produced flour with a higher protein than the second harvest (11.10% > 9.16%), which can be linked to the protein content of porang tubers as the main ingredient and the extraction and washing process (Irawan et al., 2017).
Figure 4 shows that the glucomannan content of the flour made from the first harvest was higher than from the second harvest (17.57% > 14.28%). This may result from different genetic and environmental factors that control photosynthetic rates. Moreover, in the second growing period, glucomannan might have degraded into mannan and glucose, and mannan into two molecules of mannose. Over the growing period, glucomannan, mannan, and trehalose levels decrease as they are broken down into simple sugars, i.e., glucose and mannose, to supply energy for plant growth and germination (Gusmalawati, 2021).
12
10
8
6
4
2
0
Porang Bulbss
Figure 3. Protein contents of porang flour from the first and second harvests. The letters a and b indicate a significant difference according to the post-hoc LSD test at a 5% significance level.
20
15
10
5
ba
1st Period 2nd Period
Porang Bulbs
Figure 4. Glucomannan contents of porang flour from the first and second harvests. The letters a and b indicate a significant difference according to the post-hoc LSD test at a 5% significance level.
The other part of porang plant that can be used is the axillary bulbils. However, compared to stem tubers, axillary bulbils are rarely processed into flour and are more likely used as seeds. According to Azizi and Kurniawan (2021), porang bulbils contain high glucomannan (25.78%) that can be processed into nutritional products like supplements. However, bulbils only contain 9.52% protein, which is very low. Glucomannan varies depending on plant age and the duration that porang tubers are left in storage after harvest (Azizi and Kurniawan, 2021).
Correlations between Harvest Period, Yield, Protein, and Glucomannan Content
A Pearson’s correlation test was conducted to determine the correlations between the research variables. Table 1 shows that protein and glucomannan contents strongly correlate with the harvest period. However, there are weak correlations between yield and harvest period, protein, and glucomannan content. The later the harvest period, the lower the yield, protein, and glucomannan levels. Tubers with lower yields also contain less protein and glucomannan content.
Table 1. Pearson correlation coefficients (r-values) between harvest period, yield, protein content, and glucomannan content.
|
Harvest period |
Yield (g) |
Protein content (%) |
Glucomann an content (%) | |
|
Harvest period |
1.00 |
-0.42 |
-1.00 * |
-1.00 * |
|
Yield (g) |
-0.42 |
1.00 |
0.41 |
0.42 |
|
Protein content (%) |
-1.00 * |
0.41 |
1.00 |
1.00 * |
|
Glucomann an content (%) |
-1.00 * |
0.42 |
1.00 * |
1.00 |
*) significant at a 5% significance level.
CONCLUSION
Porang stem tubers harvested in the first and second periods are similarly dark yellow. Porang flour contains glucomannan in the range of 14.28– 17.57% and protein varying from 9.16% to 11.10%, which are generally higher than those of bulbils (25.78% glucomannan and 9.52% protein). Statistically, the harvest period is strongly correlated with protein and glucomannan contents. However, yields have weak correlations with harvest
period, protein, and glucomannan content. The later the harvest period, the lower the yield, protein, and glucomannan levels.
REFERENCES
AOAC. 2012. Official Methods of Analysis of AOAC International. 19th Edition. Maryland: AOAC International Press.
Azizi, I.; and Kurniawan, F., 2021. Pengaruh Bibit Asal, Umur, dan Ukuran terhadap Kadar
Glukomanan dan Kadar Oksalat dalam Umbi Porang [Effect of Seed Origin, Age, and Size on Glucomannan Levels and Oxalate Levels in Porang Tuber]. Jurnal Sains & Seni ITS, 9 (2).
Chairiyah N.; Nunung H.; Retno M., 2014. Pengaruh Waktu Panen Terhadap Kandungan Glukomanan pada Umbi Porang
(Amorphophallus muelleri blume) Periode Tumbuh Ketiga [Effect of Harvest Time on Glucomannan Content in Porang
(Amorphophallus muelleri blume) Tuber Third Growing Period]. Research Journal of Life Science, 1(1): 37-42.
Faridah, A.; Widjanarko, S.B.;
Sutrisno, A.; dan Susilo, B., 2012.
Optimasi produksi tepung Porang dari chip Porang secara mekanis dengan metode permukaan respons [Optimizing mechanical
production of porang flour from porang chips using the response surface methodology]. Jurnal Teknik Industri, 13 (2) 158-166.
Gusmalawati, D.; Arumingtyas, E.L.;
Mastuti, R.; and Azrianingsih, R., 2021. Determination of
postharvest quality of porang (Amorphophallus Muelleri Blume)
tubers based on the dynamics of weight loss, water content and carbohydrate components for the pharmaceutical industry.
Farmacia, 69 (6) 1145–1152.
Irawan, F.; Sumual, M.F.; and Pontoh, J., 2017. Pengaruh Umur Panen Terhadap Sifat Fisik Tepung Jagung Manis (Zea mays saccharata Sturt) [Effect of Harvesting Age on Physical Properties of Sweet Corn Flour (Zea mays saccharata Sturt)]. Jurnal Teknologi Pertanian (Agricultural Technology Journal), 8 (1) 36–46.
Julianto, R.P.D., Indawan, E., and Paramita, S. 2020. Perbedaan Karakter Hasil Tiga Varietas Ubi Jalar Berdasarkan Waktu Panen [Differences in Yield
Characteristics of Three Sweet Potato Varieties Based on Harvest Time]. Jurnal Kultivasi, 19 1223– 1229.
Lintang, M.; Layuk, P.; and Joseph, G.H., 2017. Karakteristik Tepung Umbi Daluga (Cyrtosperma merkussi), Wongkai (Dioscorea
sp), Kolerea (Colocasia sp), dan Longki (Xanthosoma sp) asal Sulawesi Utara, Substitusi Terigu untuk Pangan Pokok
[Characteristics of Daluga (Cyrtosperma merkussi), Wongkai (Dioscorea sp), Cholerea
(Colocasia sp), and Longki (Xanthosoma sp) Tuber Flour from North Sulawesi, Wheat
Substitution for Staple Foods]. Jurnal Penelitian Pascapanen Pertanian, 13 (2) 83.
Minantyorini, and Somantri, I.H., 2002. Panduan karakterisasi dan evaluasi plasma nutfah Talas [Guidelines for taro germplasm characterization and evaluation].
Komisi Nasional Plasma Nutfah. p 83.
Muchtadi, T.R.; Sugiyono and Ayustaningwarno, 2013. Ilmu Pengetahuan Bahan Pangan [Food Science] (Bandung: Alfabeta).
Purwanto, A., 2014. Pembuatan Brem padat dari Umbi Porang (Amorphophallus Omcophyllus Prain) [Brem cake production from porang tubers
(Amorphophallus Omcophyllus Prain)]. Widya Warta 1 16 - 28.
Saleh N.; Rahayuningsih, St.A.; Radjit, B.S.; Ginting, E.; Harnowo, D.; Mejaya, M.J.M., 2015. Tanaman Porang pengenalan, budidaya, dan pemanfaatannya [Porang plant introduction, cultivation, and utilization]. Center for Food Crop Research and Development. Bogor.
Sari, R.S.; and Suhartati, S. (2015). Tumbuhan Porang: Prospek
Budidaya Sebagai Salah Satu Sistem Agroforestry [Porang Plants: Prospects for Cultivation as One of the Agroforestry Systems.]. Jurnal Penelitian Sosial dan
Ekonomi Kehutanan, 12(2) 97-110.
Sitompul, M.R.; Suryana, F.S.; Mahfud, M.; and Bhuana, D.S., 2018. Ekstraksi asam oksalat dari umbi porang (Amorphophallus oncophyllus) dengan pemisahan secara mekanis [Extraction of oxalic acid from porang tubers (Amorphophallus oncophyllus) by mechanical separation]. Jurnal Teknik ITS. 7(1) 135-137.
Widjanarko, S.B.; Johana, M., 2015. Analisis Metode Kolorimetri Dan Gravimetri Pengukuran Kadar Glukomanan Pada Konjak (Amorphophallus Konjac) [analysis of Colorimetric and Gravimetric Methods
for Measuring Glucomannan Levels in
Konjac (Amorphophallus Konjac).]. Jurnal Pangan dan Agroindustri, 3(4)
1584-1588.
Yuniwati, I.; Pamuji, D.R.; and Trianasari, E., 2020. Pembuatan tepung porang sebagai upaya peningkatan penjualan umbi porang di masa pandemi covid19 [Making porang flour as an effort to increase sales of porang tubers during the Covid-19 pandemic]. Jurnal Inovasi Hasil Pengabdian Masyarakat (JIPEMAS). 6 (3) 104-111.
24
Discussion and feedback