INCREASED TNF-? mRNA EXPRESSION IN PBMC CELLS INFECTED WITH HEPATITIS B VIRUS EXPOSED TO PURPLE SWEET POTATO EXTRACT (Ipomoea batatas L.)
on

ISSN: 2597-8012 JURNAL MEDIKA UDAYANA, VOL. 12 NO.12,DESEMBER, 2023
DOAJ
DIRECTORY OF OPEN ACCESS JOURNALS

Diterima: 2023-10-19 Revisi: 2023-11-08 Accepted: 25-11-2023
INCREASED TNF-α mRNA EXPRESSION IN PBMC CELLS INFECTED WITH HEPATITIS B VIRUS EXPOSED TO PURPLE SWEET POTATO EXTRACT (Ipomoea batatas L.)
Made Agus Hendrayana1
1 Department of Microbiology, Faculty of Medicine, Udayana University e-mail: agus_hendrayana@unud.ac.id
ABSTRACT
Hepatitis B Virus infection is a serious health problem throughout the world that can cause death. Purple sweet potatoes contain quite high levels of anthocyanins. Anthocyanins have been widely proven to act as immunostimulators by influencing levels of the cytokine TNF-α. This study aims to prove that purple sweet potato extract has an effect on TNF-α mRNA expression in PBMC cell cultures infected with the hepatitis B virus. The research sample was a PBMC cell culture taken from an asymptomatic chronic Hepatitis B patient. The samples from the normal saline control group were 18 wells and the treatment group with purple sweet potato extract with an anthocyanin content of 0.265 mg/100g was 18 wells. Purple sweet potato extract is made using the maceration technique. PBMCs were isolated from blood using Ficoll-Paque. PBMC cells were cultured in 24 well plates with complete RPMI 1640 medium. Examination of TNF-α mRNA expression using Relative 1-step qRT-PCR quantification. The results showed the mean difference in TNF-α mRNA expression between the treatment and control groups: 20.08 fg/µL (p<0.05), there was a significant increase in TNF-α mRNA expression. The results of this study can be concluded that administration of purple sweet potato extract in vitro can increase TNF-α mRNA expression in PBMC cultures infected with hepatitis B virus.
Kata kunci : Purple sweet potatoes, anthocyanin, TNF-α
INTRODUCTION
Hepatitis B virus infection remains high worldwide, especially in low and middle income countries. About 70% of the 257 million people living with HBV infection live in areas highly endemic for HBV infection, especially in parts of the Asian and African continents. The World Health Organization (WHO) estimates that in 2015 HBV infection caused 900,000 deaths.1
TNF-α is a proinflammatory cytokine that can inhibit the replication of certain viruses in a non-cytopathic manner without cell damage, as shown for HBV. TNF-α works independently to inhibit HBV replication. TNF-α suppresses HBV replication by activating the nuclear factor kappa-B (NF-kB) signaling pathway, especially inhibiting the formation or disrupting the stability of the HBV capsid capsule, thereby inhibiting viral replication.2,3
Purple sweet potato is a plant that is widely known in the community. This sweet potato contains the natural coloring substance anthocyanin. Purple tubers contain higher levels of anthocyanin than other colored tubers. Purple sweet potato contains anthocyanin which varies between 110mg/100gr to 210mg/100gr of fresh tubers, while the anthocyanin content in the water extract of purple sweet potato tubers is 140.23 - 147.0 mg/mL.4
Anthocyanin, which is one of the phenolic compounds, has been studied for its effect on TNF-α production. Anthocyanins can induce the production of TNF-α, and can significantly reduce the production of Nitric Oxide (NO) thereby reducing oxidative stress in the anti-inflammatory process.5
This study aims to prove that purple sweet potato extract can increase the expression of messenger ribonucleic acid (mRNA) TNF-α in peripheral blood mononuclear cell (PBMC) cultures, so that it can provide information for consideration that purple sweet potato extract can be used to help manage HBV infection and can be used as a reference material for further research.
ANTHOCYANINS AND TNF-α
The immune system against HBV infection is quite complex and involves the innate and adaptive immune systems. Innate and adaptive immune system responses play a role in the development of hepatitis B. Acute HBV infection can recover spontaneously through the production and activation of the immune response of antibodies, Kupler cells, natural killer (NK) cells, CD4/CD8 T cells, interleukin-2 (IL-2), interferon-gamma (IFN-γ), and TNF-α. 6,7
TNF has an important role in the inflammatory process and regulation of the immune system as well as cell differentiation and proliferation. TNF plays a role in virus clearance. There are several pathways by which TNF exerts antiviral activity. TNF can reduce viral entry into hepatocytes. TNF also reduces viral replication and disrupts nucleocapsids by disrupting covalently closed circular DNA (cccDNA) through activation of apolipoprotein B editing complex (APO-BEC3) deaminase which leads to destruction of HBV cccDNA.8
Table 1 TNF-α mRNA Gene Primers. 12
|
Target |
Sequence 5’-3’ |
|
Forward Gen mRNA TNF-α |
CCG AGG CAG TCA GAT CAT CTT |
|
Reverse Gen mRNA TNF-α |
AGC TGC CCC TCA GCT TGA |
Amplification is carried out on a thermal cycler machine with the following protocol :
|
Table 2 Real Time PCR Amplification Protocol Conditions 13 Step Temperature Duration Cycle cDNA synthesis 42oC 5 minutes hold RT inactivation 95oC 5 minutes hold Denaturation 95oC 3 seconds 40 Annealing 60oC 20 seconds |
The bioactive component anthocyanin is a natural coloring substance in food which is categorized as an antioxidant. Food ingredients such as fruit, vegetables, roots, tubers, nuts and cereals with a bluish or purplish color contain quite high levels of anthocyanin. Anthocyanins have a structural formula with a phenolic structure so they can transfer hydrogen atoms from hydroxyl free radicals.9,10,11
Anthocyanins induce the production of TNF-α and act as modulators of the immune response in activated macrophages. Anthocyanins induce production of TNF-α in macrophage cells. The mechanism of inhibitory and stimulatory activity of flavonoids on TNF-α production can result from both transcriptional and post-transcriptional processes. Anthocyanins induce the production of TNF-α in a dose-dependent manner. At concentrations of 250 μM and 500 μM, most anthocyanins can significantly induce the production of TNF-α.5
MATERIALS AND METHODS
This research is an in vitro laboratory experimental study using PBMC cell culture with a Randomized Pre and Post Test Control Group Design. The
research was conducted at the Integrated Biomedical Laboratory Unit, Faculty of Medicine, Udayana University.
The sample used in this study was PBMC cell culture taken from one patient suffering from asymptomatic chronic Hepatitis B in accordance with sample criteria and procedures. This study used a sample of 3,263.7 cells/µL PBMC cells or 7,637,058 cells for 36 well plates for PBMC cell culture. The 36 wells were then divided into two groups, namely the control group and the treatment group randomly. For each group, 18 PBMC culture wells were obtained with the following details: The control group consisted of 18 normal saline groups. The treatment group consisted of a group of purple sweet potato tuber water extract with a dose of 50 µL in 18 wells.
The anthocyanin content in purple sweet potato extract is 5.3 mg/100gr. In this study, 50 µL of purple sweet potato extract was treated in each PBMC culture well with an anthocyanin content of 0.265 mg/100gr. RNA isolation was carried out using a commercial kit. The PCR method used is Relative 1-step qRT-PCR quantification using a commercial kit using the following primer sequence:
Amplification was carried out on a MyGo Mini Real
Time PCR thermal cycler to obtain Ct (cycle threshold) RESULTS
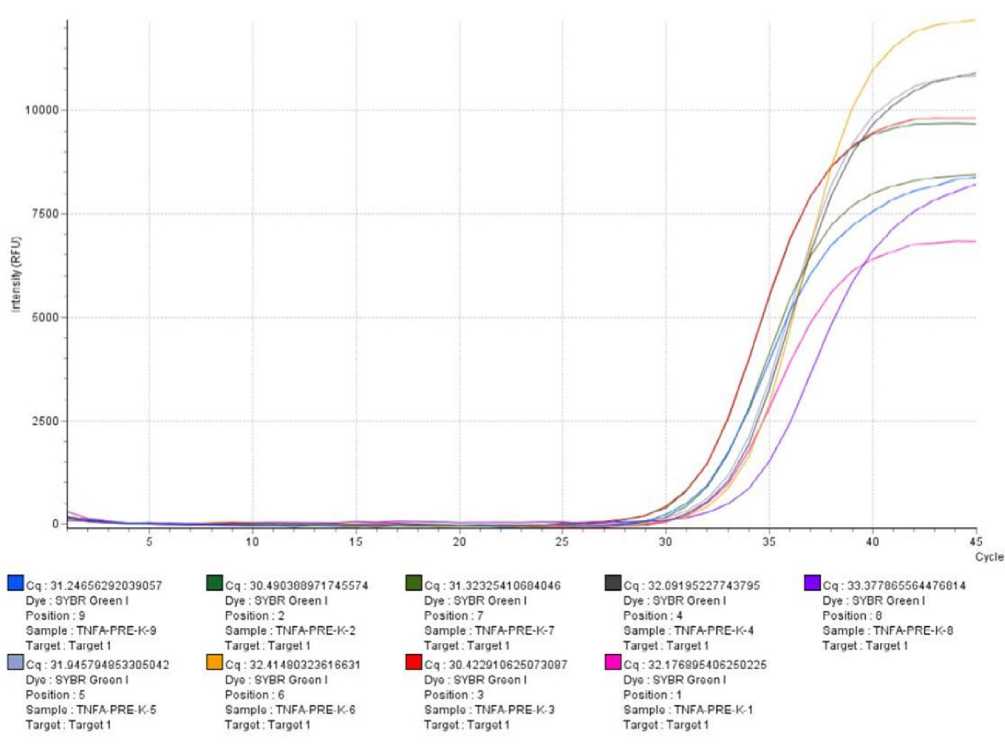
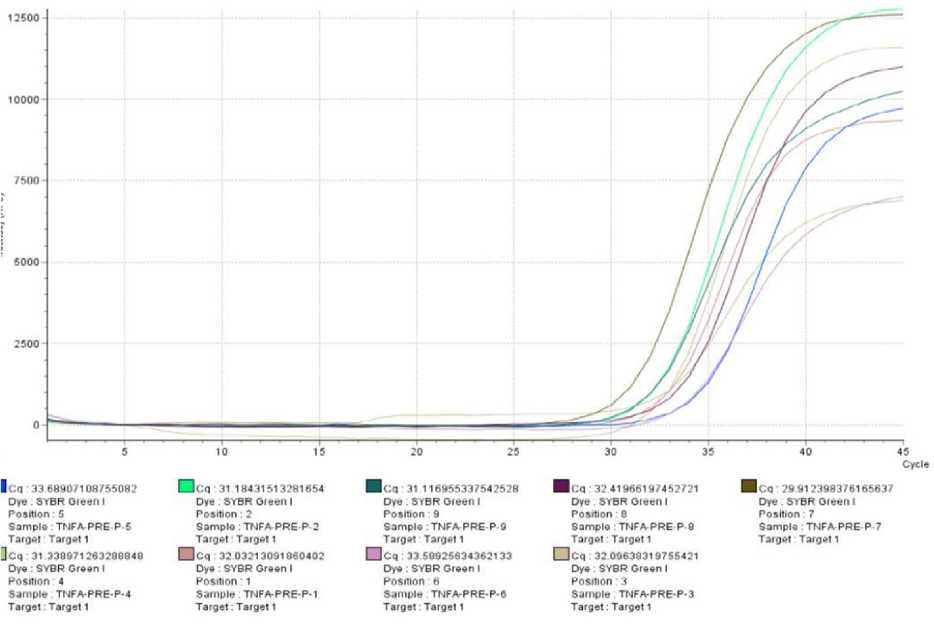
data and then interpolarized using a Ct serial dilution TNF-α mRNA expression in PMBC cell cultures is shown
standard curve. TNF-α mRNA expression data was in Figure 1
obtained in numbers in units of fg/µL and then compared
for analysis.
IOOOO
2500
7500
5000
Hcq: 31.24656292039057
Dye: SYBR Oreen 1
Position . 9
Sample: TNFMsRE-K-S
Target Target 1
Hcq 31.045704653305042
Dyo: SYBR Groon I
Position: 5
Sompk TNFA PRE K 5
Target Target 1
Hcq 32.09195227743795
Dye : SYBR Oreen I
Position: 4
Sample TNFA-PRE-K-4
Target Target 1
Hcq 32.176805406250226
DyG : SYBR Greon I
Position: 1
Sample TNFA PRE E 1
Target Target 1
Hcq: 33.377065564476614
Dye SYBR Oreen I
Position θ
Sample TNFA-PRE-K-G
Target Target 1
Hcq: 30.490360971745574
Dye : SYBR Oreen I
Position; 2
Sample : TNFA-PRE-K-Z
Target Target 1
Ocq 32 41480323616631 Dye : SVBR Green I
Positron: 6
Sample:TNFA-PRE K 6
Target Target 1
Hcq : 31.3232541066404G Dye SYBR Oreen I Position: 7
Sample TNFA-PRE-K-7
Target Target 1
Hcq : 30 4 22010625073097 Dyo SYBR Greon I
Position: 3
Sample TNFAPRE∙k∙3
Target Target 1
45
Cycle
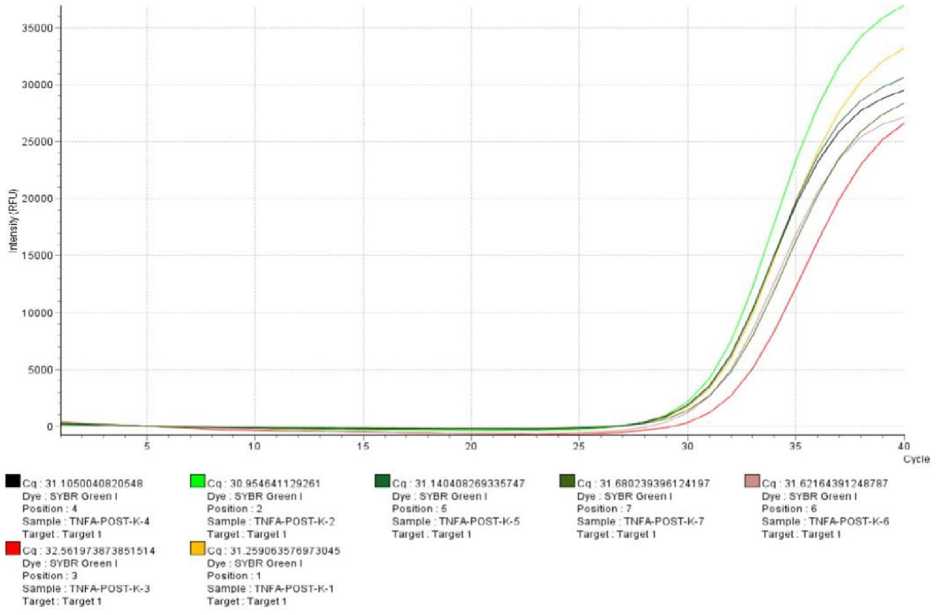
Hcq : 32.75328001 71 2689
Dγ⅛∣ SYBR Giwqii I
Position . 5
SAmpte TnFA-POST-K-S
Target Target 1
Hcα 32.571 31 874562753 Dyw SYBR Green ∣ Position . t
Sample TNFa-PoST-K-B Target Target t
Figure 1 Curve of quantitative real time PCR (qRT-PCR) amplification results of TNF-α mRNA expression from research samples
To determine the difference in the effect of treatment on TNF-α mRNA expression after observation between samples treated with purple sweet potato extract (treatment group) and samples that did not receive treatment (control group), statistical analysis was carried out using the
independent t test because the TNF-α mRNA expression data in the treatment group and control group were normally distributed. The results of statistical analysis tests of differences in TNF-α mRNA expression in the two groups are presented in Table 3
Table 3 Differences in TNF-α mRNA expression between the two groups after observation
|
TNF-α mRNA Expression (fg/µL) | |||||
|
Group |
N |
Mean ± SD |
Mean difference |
CI 95% (Min-Max) |
p* |
|
Treatment |
9 |
17,96 ± 20,65 |
20,08 |
-0,54 - 40,72 |
0,05 |
|
Control |
9 |
-2,13 ± 20,64 | |||
SD: standard deviation; CI: confidence interval; Min: minimum; Max: maximum * significant on p<0,05
In Table 3 you can see the results of the independent t test analysis of the differences in TNF-α mRNA expression in the treatment group and control group. In the treatment group, the mean difference in TNF-α mRNA expression was 17.96 ± 20.65 fg/µL, while in the control group it was -2.13 ± 20.64 fg/µL. In the treatment group and control group, the mean difference in TNF-α mRNA expression was found to be 20.08 fg/µL. These results indicate that there is a significant difference in TNF-α mRNA expression between the treatment group and the control group, where the treatment group had higher TNF-α mRNA expression than the control group (p<0.05).
DISCUSSION
This study showed that there was a significant difference in TNF-α mRNA expression in the treatment group and the control group, where the treatment group had higher TNF-α mRNA expression than the control group. These results confirm that the anthocyanin compound content in purple sweet potato extract can increase TNF-α mRNA expression.
Several natural extracts from plants have been reported to increase the expression of TNF-α. Research by Chang et al. (1994) showed that natural ingredients from plant extracts with the compounds fisetin and quercetin
(flavonol), daidzein (isoflavone) and flavonoids can play a role in improving the body's immune system and antiviral activity by increasing the expression of TNF-α. 14,15
The polyphenolic compound resveratrol from red grapes significantly increased TNF-α mRNA expression along with increased TNF-α secretion in LPS-activated RAW 264.7 macrophage cells. The flavonoid substance flavone-8-acetic acid (FAA) has been shown to induce TNF-α secretion in J774 cells.16
This research uses purple sweet potato extract which contains anthocyanin compounds which can increase TNF-α mRNA expression in PBMC cell cultures. Anthocyanins are a group of bioactive flavonoids. Anthocyanins showed significant effects on the production and induction of TNF-α. Production of TNF-α by macrophages can occur in different ways.5
Other research showing that anthocyanins from natural extracts can increase TNF-α mRNA expression is shown by research conducted by Roth et al. (2014) where their research shows that exposure to anthocyanins from bilberry extract can increase TNF-α-stimulation mRNA expression and secretion NF-κB target genes.17
Anthocyanin is a large molecule that has high solubility in water, it has been proven that when cells are exposed to anthocyanin, the anthocyanin actually crosses the cell membrane and its presence can be detected inside the cell. Anthocyanins are water soluble and sufficiently lipophilic for spontaneous penetration into cells through the lipid bilayer.18,19
The absorption of anthocyanins into cells is influenced by sugar groups. Anthocyanin transporters are glucose transporters, because anthocyanins have sugar parts, especially glucose residues. Flavonoid glucosides such as quercetin-3-glucose can enter cells by the presence of glucose residues using the GLUT2 hexose transporter. Exposure of cells to anthocyanins triggers the expression of the GLUT2 transporter which plays a role in the absorption of anthocyanins into cells through the cell membrane. 20,21,22
Anthocyanins show a significant effect on increasing TNF-α mRNA expression and TNF-α secretion in macrophage cells through activating the NF-κB pathway by stimulating the secretion of NF-κB target genes. Anthocyanin-induced TNF-α expression may occur in different ways depending on time and dose. 23,24,25,26
TNF is essential for viral clearance. There are many pathways that TNF uses to carry out antiviral activity. First, TNF reduces viral entry into hepatocyte cells. The TNF pathway has great clinical relevance, where TNF is a key mediator in the immune system for clearance of chronic HBV. Increasing TNF levels will likely eradicate the virus.8,27
CONCLUSIONS AND SUGGESTIONS
Based on this research, it can be concluded that purple sweet potato extract can increase TNF-α mRNA expression in PBMC cultures of asymptomatic chronic hepatitis B sufferers compared to controls.
It is necessary to conduct research on the direct influence of anthocyanin compounds from purple sweet
potato extract on components in the structure of the HBV virus through molecular pathways that can be influenced. This will make it possible to determine and analyze the role of anthocyanins from purple sweet potato extract as antivirals directly.
REFERENCE
-
1. WHO. Progress and challenges with hepatitis B virus (HBV) elimination in Guideline : Prevention of mother-to-child transmission of hepatitis B virus: guidelines on antiviral prophylaxis in pregnancy. World Health Organization; 2020.Chapter 1.1: 1-2
-
2. Guidotti, L.G., dan Chisari, F.V. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65-91.
-
3. Biermer, M., Puro, R., dan Schneider, R.J. Tumor Necrosis Factor Alpha
Inhibition of Hepatitis B Virus Replication Involves Disruption of Capsid Integrity
through Activation of NF-kB. Journal of Virology.2003;77(7): 4033–4042.
-
4. Suprapta, N.D., Antara, M., Arya, N., Sudana, M., Duniaji, A.S. dan Sudarma, M. Penelitian Peningkatan Kualitas dan Diversifikasi Penggunaan Umbi-Umbian
sebagai Sumber Pangan Alternatif di Bali. Fakultas Pertanian UNUD. 2004.
-
5. Wang, J., dan Mazza, G. Effects of Anthocyanins and Other Phenolic
Compounds on the Production of TNF α in LPS/IFN-γ-Activated RAW 264.7
Macrophages. J. Agric. Food Chem. 2002;50(15).
-
6. Mankgopo, M., Kgatle, Setshedi, M. Immunopathogenesis of Hepatitis B
Virus Infection and Related Complications. Europian Med. Journal Hepatology. 2016;
4(1):84-92.
-
7. Rehermann, B. Pathogenesis of chronic viral hepatitis: differential roles of T
cells and NK cells. Nat Med. 2013;19(7):859-68.
-
8. Valaydon, Z., Pellegrini, M., Thompson, A., Desmond, P., Revill, P., dan Ebert, G. The role of tumour necrosis factor in hepatitis B infection: Jekyll and Hyde. Clinical & Translational Immunology. 2016;5:e115.
-
9. Santoso, Arief, dan Estiasih, T. Kopigmentasi Ubi Jalar Ungu (Ipomoea Batatas var. Ayamurasaki) dengan Kopigmentasi Na-Kaseinat dan Protein Whey serta Stabilitasnya terhadap Pemanasan. Jurnal Pangan dan Agroindustri. 2014;2(4):121-127.
-
10. Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative
damage. Am J Clin Nutr. 2003;78(3): 570s-578s
-
11. Tall, J.M., Seeram, N.P., Zhao, C., Nair, M.G., Meyer, R.A., dan Raja, S.N.
Tart cherryanthocyanins suppress inflammation-induced pain behavior in rat.
Behavioural brain research.2004;153(1):181-8.
-
12. Carneiro, J.R.M., Fuzii, H.T., Kayser, C., Alberto, F.L., Soares, F.A., Sato, E.I., Andrade,L.E.C. IL-2, IL-5, TNF-a and IFN-c mRNA expression in epidermalkeratinocytes of systemic lupus erythematosus skin lesions. CLINICS. 2011;66(1):77-82.
-
13. Nasiri,M., Geramizadeh, B., S. H.Nabavizadeh, S.H., Male-Hosseini, S.A., Karimi,M.H.,Saadat, I. mRNA Expression of Interferon Regulatory Factors during Acute Rejection of Liver
Transplants in Patients with Autoimmune
Hepatitis. Int J OrgTransplant Med. 2018; 9(1)
-
14. Chang, C., Hsu, F., Lin, J. Inhibitory effects of polyphenolic catechins from
Chinese green tea on HIV reverse transcriptase activity. J. Biomed. Sci. 1994;1: 163-166.
-
15. Seo, D.J., dan Choi, C. 2017.Inhibitory mechanism of five natural flavonoids against murinenorovirus. Phytomedicine.
-
16. Park, Y. C., Rimbach, G., Saliou, C., Valacchi, G., Packer, L. Activity of
monomeric, dimeric, and trimeric flavonoids on NO production, TNF- α secretion,
and NF-κB-dependent gene expression in RAW 264.7 macrophages. FEBS Lett.2000; 464, 93-97.
-
17. Roth, S., Spalinger, M.R., Müller, I., Lang, S., Rogler, G., Scharl, M. Bilberry-derived anthocyanins prevent IFN-γ-induced pro-inflammatory signalling and cytokine secretion in human THP-1 monocytic cells. Digestion. 2014;90(3):179-189.
-
18. McGhie,T.K., and Walton,M.C. The
bioavailability and absorption of
anthocyanins: Towards a better understanding. Mol. Nutr. Food Res.2007;51:702 – 713
-
19. Tarahovsky, Y.S., Kim, Y.A., Yagolnik, E.A., Muzafarov. E.N. Flavonoid–
membrane interactions: Involvement of
flavonoid–metal complexes in raft signaling.
Biochimica et Biophysica Acta. 2014;1838;1235– 1246
-
20. Lazze, M.C., Savio, M., Pizzala, R., Cazzalini, O., Perucca, P., Scovassi., A.I.,
Stivala, L.A., dan Bianchi, L. Anthocyanins
induce cell cycle perturbations
and apoptosis in different human cell lines. Carcinogenesis.2004.25(8):1427-1433
-
21. Faria, A., Pestana, D., Azevedo, J., Martel, F., Freitas, V., Azevedo, I., Mateus, N., Calhau,C. Absorption of anthocyanins through intestinal epithelial cells – Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009; 53:1430– 1437
-
22. Kamiloglu, S., Capanoglu, E., Grootaert, C., Camp, J.V. Anthocyanin
Absorption and Metabolism by Human Intestinal Caco-2 Cells—A Review. Int. J. Mol. Sci.2015;16:21555-21574
-
23. Bao,W., Wang,Y., Fu,Y., Jia,X., Li,J., Vangan,N. mTORC1 Regulates
Flagellin- Induced Inflammatory Response in Macrophages. PLoS ONE.2015;10(5): e0125910. doi:10.1371/journal.pone.0125910
-
24. Liobikas, J., Skemiene, K., Trumbeckaite, S., Borutaite, V. Anthocyanins in
cardioprotection: a path through mitochondria. Pharmacological Research. 2016.Accepted Manuscript.
http://dx.doi.org/doi:10.1016/j.phrs.2016.03.036
-
25. Schusterová P, Eliašová A, Mudroňová D, Csank T, and Tkáčiková Ľ. 2017. In vitro study of
immunomodulatory activities of purified
anthocyanins-rich berry extract prepared from Sambucus nigra. 2017.
https://www.researchgate.net/publication/319103 789
-
26. Huang, W., Hutabarat R.P., Chai, Z., Zheng, T., Zhang, W., and Li, D.
Antioxidant Blueberry Anthocyanins Induce Vasodilation via PI3K/Akt Signaling
Pathway in High-Glucose Induced Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2020;21(1575)
https://doi.org/10.3390/ijms21051575
-
27. Watashi, K., Liang, G., Iwamoto, M., Marusawa, H., Uchida, N., Daito, T. Interleukin-1 and tumor necrosis factor-alpha trigger restriction of hepatitis B virus infection via a cytidine deaminase activation-induced cytidine deaminase (AID). JBiol Chem.2013;288:31715–31727.
http://ojs.unud.ac.id/index.php/eum
doi:10.24843.MU.2023.V12.i12.P14
111
Discussion and feedback