CINNAMON (Cinnamomum burmanii) ETHANOL EXTRACT HEPATOPROTECTIVE ACTIVITY AGAINST ISONIAZID INDUCED RATS LIVER HISTOPATHOLOGY
on

ISSN: 2597-8012 JURNAL MEDIKA UDAYANA, VOL. 13 NO.02, FEBRUARI, 2024


Received: 2023-12-28 Revision: 2023-10-28 Accepted: 25-12-2023
CINNAMON (Cinnamomum burmanii) ETHANOL EXTRACT HEPATOPROTECTIVE ACTIVITY AGAINST ISONIAZID INDUCED RATS LIVER HISTOPATHOLOGY
Evi Sovia1*, Henny Juliastuti2, Siti Julaeha3, Welly Ratwita1
1Departemen Farmakologi, Fakultas Kedokteran Universitas Jenderal Achmad Yani, Cimahi, Indonesia 2Departemen Biokimia, Fakultas Kedokteran Universitas Jenderal Achmad Yani, Cimahi, Indonesia 3Program Studi Kedokteran, Fakultas Kedokteran Universitas Jenderal Achmad Yani, Cimahi, Indonesia e-mail: evi.sovia@lecture.unjani.ac.id
ABSTRAK
Isoniazid (INH) an anti-tuberculosis drug that can cause drug-induced liver injury (DILI). Cinnamon (Cinnamomum burmanii) has benefits as an antioxidant and potentially as a hepatoprotective. The study aims to find out the hepatoprotective activity of cinnamon ethanol extract against the liver histopathology of male rats induced by INH. The study is a laboratory experiment with posttest only control group design with a total sample of 25 rats. The animals were divided into five groups: the negative control group given aquabidest, the positive control group induced by INH at doses of 200mg/kg, and the three treatment groups that were induced by the INH with a dose of 200 mg/kg and were given cinnamon ethanol extracts with doses 100, 200, and 400 mg/kg for 14 days. At the end of the study, the animal was sacrificed and the liver was taken for preparation. The histopathology scores of liver cell damage in rats used HAI-Knodell scores. The results of the Knodell score were analyzed using the One Way Anova and Post Hoc Duncan tests. The results of the study showed that the highest average liver damage score was found in the positive control group (2,80±1,64), while the lowest average score was in the negative control group (0,40±0,55). Liver damage scores in the groups given cinnamon ethanol extract in doses of 100, 200, and 400 mg/kg were 1.20±0.84; 0.80±0.84 and 0.80± 0.84, respectively. There were significant differences between the positive control group and the treatment group (p<0,05), but there was no significant difference between the treatment groups. Cinnamon ethanol extract has hepatoprotective activity with an effective dose of 100 mg/kg.
Keywords: Cinnamomum burmanii, hepatoprotection, isoniazid
INTRODUCTION
Drug-induced liver injury (DILI) is a challenging diagnosis for doctors and has been studied extensively. Every year, DILI receives increasing attention from doctors, researchers, and patients because it poses significant risks to patient health and healthcare costs. Drug-induced liver injury caused by anti-tuberculosis drugs continues to be a challenge in the management of tuberculosis (TB) and is a major reason for discontinuation of treatment worldwide.1
TB remains the 10th largest cause of death in the world. It is estimated that a quarter of the world's population is infected with Mycobacterium tuberculosis, with an estimated 10 million new cases and 1.5 million deaths in 2018. Isoniazid (INH) is the most clinically successful and extensively studied TB drug ever developed. INH is bactericidal that inhibits the synthesis of mycolic acid in bacterial cell walls, and is effective against intra and extracellular organisms. Despite the proven and strong effectiveness of INH, INH has long been known as
hepatotoxic and can cause liver failure.2,3,4,5 Liver disorder is a critical condition, and there is currently no satisfactory treatment for it.
Cinnamon (Cinnamomum zeylanicum) is widely used as a spice around the world, originating in Southeast Asia and South America. Currently available in two main varieties namely, Cassia and Ceylon (Cinnamon cassia dan Cinnamomum zeylanicum).6 A number of studies have shown the presence of an active biological compound in cinnamon, namely cinnamaldehyde. These compounds are found in different ratios in all parts of the plant. The bioactive compounds found in cinnamon can affect the human body in various ways and can be applied to treat a wide range of diseases and metabolic disorders. There is sufficient evidence that cinnamon has a positive impact on human health, such as antimicrobial, antidiabetic, hepatoprotective, neuroprotective, cardioprotective, immunomodulatory and anticancer.7
Previous research has shown that administration of cinnamon provides potential therapeutic applications in
acute liver and kidney injury caused by acetaminophen and may have therapeutic potential in oxidative stress-related diseases. Cinnamon extract has also been proven to improve and reduce levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in rat liver induced by paracetamol and carbon tetrachloride.6,8,9
Previous research generally used acetaminophen as a hepatotoxic inductor, whereas the most common cause of DILI was antituberculosis drugs. This study aims to determine the hepatoprotective activity of ethanol extract of cinnamon (Cinnamomum burmanii) on the histopathological features of the liver of male Wistar rats induced by INH.
MATERIAL AND METHOD
This research is an experimental study to determine the hepatoprotective activity of ethanol extract of cinnamon (Cinnamomun burmanii) on liver histopathology using a true experimental post-test only control group design. The cinnamon used in this research was obtained from the Manoko Medicinal Plant Experimental Garden, Lembang, Bandung and the part used was cinnamon bark which was still intact, fresh and not rotten.
Equipment used includes oven, analytical scale, blender, beaker glass, stirring rod, macerator, filter paper, rotary evaporator, scale, rat cages, husks, sondes, 1 cc and 5 cc disposable syringes, tissue cassettes, base molds, microtomes, water bath, object glass, and cover glass. The materials used include cinnamon bark, aquabidest, ethanol 96%, standard feed (rat bio), INH, physiological NaCl, alcohol 70%, 80%, 95%, 100%, hematoxylin-eosin solution, 10% formalin solution, ethylene, liquid paraffin, and xylol solution.
The experimental animals used in this research were 25 male Wistar rats obtained from Biofarma. Experimental animals were divided into control groups and treatment groups. The control group consisted of positive control induced by INH 200mg/kg and negative control given aquabidest. The treatment groups were divided into three groups that given cinnamon ethanol extract with doses of 100, 200, and 400 mg/kg. This research was conducted at the Pharmacology Laboratory and Animal Laboratory, Faculty of Medicine, Jenderal Achmad Yani University from July to December 2022. This research has received an ethical clearance statement from the Health Research Ethics Commission, Faculty of Medicine, Jenderal Achmad Yani University with number 021/UH2.10/2022.
Plant extraction
The maceration method is used to make ethanol extract of cinnamon bark. A total of 2.5 kg of cinnamon bark was cleaned. Then, the cinnamon bark was cut into smaller pieces, and put in the oven at a temperature of 60oC for two days until dry. The dry cinnamon bark then weighed again to determine its weight, then chopping and grinding the cinnamon bark using a milling
machine until 1 kg of cinnamon powder is obtained. 300 grams of cinnamon powder was divided equally into three Erlenmeyer flasks and 900 ml of 96% ethanol solvent was added which was divided equally into the three Erlenmeyer flasks. The solution was stirred for 30 minutes, then the results were macerated for three days at room temperature. The solution that has been macerated will form macerate which is then filtered using filter paper. Next, evaporation is carried out using a rotary evaporator at a temperature of 90oC, so that a thicker cinnamon ethanol extract is obtained.10
Experimental animal treatment
A total of 25 mice were divided into five groups. Each group consisted of five male Wistar rats. The experimental animals were acclimatized for 7 days by being placed on a bed of husks, and given standard feed (rat bio) 20-25g/head/day and drinking water ad libitum. The animals experiment were weighed and given treatment for 14 days based on each group as follows:
-
1. Group 1 (negative control): given standard feed 20-25gr/head/day, drinking water ad libitum, and aquabidest
-
2. Group 2 (positive control): given standard feed 20-25gr/head/day, drinking water ad libitum, aquabidest, and INH dose 200mg/kgbb/day
-
3. Group 3 (treatment 1): given standard feed 20-25g/head/day, drinking water ad libitum, ethanol extract of cinnamon at a dose of 100mg/kgbb/day, and INH at a dose of 200mg/kgbb/day
-
4. Group 4 (treatment 2): given standard feed 20-25g/head/day, drinking water ad libitum, ethanol extract of cinnamon at a dose of 200mg/kgbb/day, and INH at a dose of 200mg/kgbb/day
-
5. Group 5 (treatment 3): given standard feed 20-25g/head/day, drinking water ad libitum, cinnamon ethanol extract dose 400mg/kgbb/day, and INH dose 200mg/kgbb/day
At the end of the study, the mice were sacrificed and the liver organs were removed to make liver tissue preparations.
Histopathological Examination
Tissue sections were fixed in (1.85% formaldehyde, 1% acetic acid) for 1 week at room temperature, dehydrated with graded ethanol and embedded in Paraffin. Sections (5μm thickness) were deparaffinized with xylene and stained with H (Hematoxylin) and E (Eosin) stains. Observations using a light microscope with a magnification of 100 and 400x on 25 microscopic preparations, observations of the rat liver were carried out in all areas of the preparation, especially around the edges of the central vein and portal vein, which were carried out in the three zones according to the concept of dividing microscopic
zones of the liver. The scoring system used is the Knodell Score System (HAI).11
The research data were then analyzed using Anova and presented in the form of tables and images accompanied by descriptive explanations in the form of text. Universitas Jenderal Achmad Yani dengan nomor 021/UH2.10/2022.
RESULTS
The histopathological structural changes observed were acute injuries because the treatment was only given for 14 days. Acute injuries were found such as parenchymatous degeneration, focal necrosis, portal inflammation found in the positive control group and treatment group with different extents of damage from each group, observation of preparations was carried out in each preparation area.
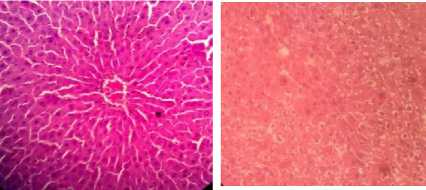
The histological examination of the negative control group which was only given aquabidest, showed that the liver structure was normal, the hepatocytes were seen arranged in rows to form radially arranged plates with the central vein in the middle, the cell membrane and cell nucleus of the hepatocytes were intact (Figure 1A).
The histological examination in the positive control group that given INH at a dose of 200mg/kg showed liver cell damage in the form of parenchymatous degeneration which was at a moderate level in around 2/3 of the liver lobules (score 1-3) accompanied by mild portal inflammation (score 0-1), focal necrosis in several places in the liver lobules (score 0-1) and there was no bridging necrosis or fibrosis found because the treatment was only for 14 days (Figure 1B).
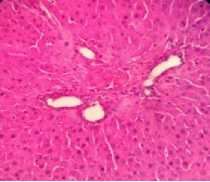
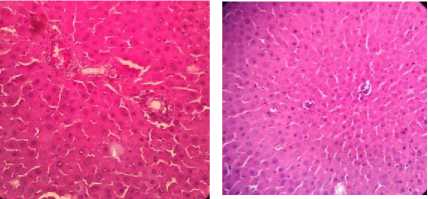
The results of histological examination of the treatment group induced by INH 200mg/kg and given cinnamon extract at doses of 100, 200, and 400mg/kg showed a reduction in liver cell damage in rats. In the group given cinnamon extract at a dose of 100mg/kg, liver damage was still in a mild degree, visible signs of damage in the form of mild parenchymatous degeneration (score 0-1), mild portal inflammation (score 0-1), focal necrosis only in a few places (score 0-1) (Figure 1C). In the group given cinnamon extract at a dose of 200mg/kg there was mild parenchymatous degeneration in several places (score 0-1), mild portal inflammation (score 0-1), and mild focal necrosis (score 0-1) (Figure 1D). Meanwhile, for the treatment group given cinnamon extract at a dose of 400mg/kg, there was mild parenchymatous degeneration (score 0-1), mild portal inflammation (score 0-1) and no focal necrosis was found (Figure 1E).
The highest average score for level of liver damage was found in the positive control group (2.80±1.64) which was only induced by isoniazid at a dose of 200 mg/kg, while the lowest average score was found in the negative control group which was only given aquabidest (0.40±0.55). Whereas in the group given cinnamon extract at doses of 100, 200, and 400mg/kg, the mean scores were found are 1.20±0.84, 0.80±0.84, and 0.80± 0.84 respectively (Table 1).
Thus, in the group treated with ethanol extract of cinnamon (Cinnamomum burmanii) at doses of 100,200, and 400mg/kg, there was a reduction in rat liver damage when compared with the positive control group.
A B
C D
E
Figure 1. Histological examination of a cross-section of rat liver, after 14 days of treatment with HE staining (400x magnification). A Control (aquabides); B. (INH 200mg/kg); C. (INH 200mg/kg + cinnamon ethanol extract 100mg/kg); D. (INH 200mg/kg + cinnamon ethanol extract 200mg/kg); E. (INH 200mg/kg + cinnamon ethanol extract 400mg/kg).
The results of Duncan's post hoc test showed that there was a significant difference between the positive control group and the treatment group, which means that administration of cinnamon extract could reduce INH-induced liver damage. Meanwhile, there were no significant differences between treatment groups, which means that with which means that increasing the dose does not cause an increase in the effect.
Tabel 1. Mean liver damage score of male Wistar rats treated with ethanol extract of cinnamon and induced by isoniazid
|
Groups |
Mean ± SD |
F(Anova) |
p value *) |
|
Negative |
0,40 ± 0,55 | ||
|
control | |||
|
Positive |
2,80 ± 1,64 |
22,919 |
0,000 |
|
control | |||
|
Cinnamon |
1,20 ± 0,84 | ||
|
100mg/kg | |||
|
Cinnamon |
0,80 ± 0,84 | ||
|
200mg/kg | |||
|
Cinnamon |
0,80 ± 0,84 | ||
|
400mg/kg |
Note: One Way ANOVA; *p<0,05 (significan difference)
DISCUSSION
Liver cell damage in the positive control group induced by INH showed that INH was hepatotoxic. Hepatotoxicity due to INH is caused by free radical reactions from the metabolism of isoniazid in the liver in the form of hydrazine and acetylhydrazine. Reactive metabolites (acetylhydrazine and hydrazine) will trigger acetylation of macromolecules which causes protein binding in the liver and a decrease in glutathione activity which is a detoxifier of reactive oxygen species (ROS).2,4,12
The results of this study support previous research which showed the hepatotoxic effect of INH which was characterized by increased levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), whereas in this study the parameters were liver cell damage observed microscopically.13
In this study, the treatment group experienced a significant reduction in the number of inflammatory cells and liver cell degeneration, this occurred due to the influence of cinnamon ethanol extract as an antioxidant which can counteract the effects of free radicals due to isoniazid induction. Previous research has proven that cinnamon has very strong antioxidant activity in vitro, contains high amounts of total phenolics and total flavonoids so it has the potential to be used as a food additive (antioxidant) in the food and pharmaceutical industries.14 Cinnamon is also a plant that has the greatest hepatoprotective activity. 15
The protective effect of cinnamon ethanol extract against liver cell damage by isoniazid is due to the active compounds contained in cinnamon including flavonoids, tannins, essential oils, alkaloids which can provide a hepatoprotective effect. Flavonoids are found in many plant tissues and can act as antioxidants. The antioxidant activity of flavonoids originates from their ability to donate hydrogen atoms or through their ability to chelate metals.16
Flavonoids can also inhibit the activity of cyclooxygenase and lipoxygenase enzymes which are inflammatory mediators by inhibiting the release of arachidonic acid which is a chemotactic component. This function is able to protect liver cells from exposure to the anti-tuberculosis drug INH.15
This research also supports previous research which shows that cinnamon extract has a hepatoprotective effect as evidenced by the decrease in ALT and AST levels in rats induced by paracetamol.8,6
Of the three doses tested in this study, there were no significant differences, so the recommended dose is the lowest dose, that is 100mg/kg. There is a phenomenon that increasing the dose is not linear to increase the therapeutic effect. Therapeutic effects that are not in line with an increase in the dose of the drug given are known as nonmonotomic dose-response relationship (NMDR).17 The effective dose in this study is smaller than the previous study which used a dose of 200mg/kg.6
CONCLUSION
Cinnamon ethanol extract has a hepatoprotective effect.
ACKNOWLEDGMENT
The author would like to thank the Institute for Research and Community Service (LPPM) at Jenderal Achmad Yani University for providing funding for this research through a Competitive Grant.
REFERENCES
-
1. Clinton JW, Kiparizoska S, Aggarwal S, Woo S,
Davis W, Lewis JH. Drug-Induced Liver Injury: Highlights and Controversies in the Recent
Literature. Drug Saf. 2021;44(11):1125-1149.
doi:10.1007/s40264-021-01109-4
-
2. Lei S, Gu R, Ma X. Clinical perspectives of
isoniazid-induced liver injury. Liver Res. 2021;5(2):45-52. doi:10.1016/j.livres.2021.02.001
-
3. Singh A, Prasad R, Balasubramanian V, Gupta N,
Gupta P. Prevalence of adverse drug reaction with first-line drugs among patients treated for
pulmonary tuberculosis. Clin Epidemiol Glob Heal. 2015;3:S80-S90. doi:10.1016/j.cegh.2015.10.005
-
4. Singh D, Cho WC, Upadhyay G. Drug-induced liver
toxicity and prevention by herbal antioxidants: An Overview. Front Physiol. 2016;6(JAN):1-18.
doi:10.3389/fphys.2015.00363
-
5. Wesnawa MADP, Kusmiati T. Drug Induced
Hepatitis pada Tuberkulosis Paru dengan Multisite Tuberkulosis Ekstraparu. J Respirasi. 2020;5(2):34. doi:10.20473/jr.v5-i.2.2019.34-40
-
6. Hussain Z, Khan JA, Arshad A, Asif P, Rashid H,
Arshad MI. Protective effects of Cinnamomum zeylanicum L. (Darchini) in acetaminophen-induced
Page 9
oxidative stress, hepatotoxicity and nephrotoxicity 12. in mouse model. Biomed Pharmacother.
2019;109(August 2018):2285-2292.
doi:10.1016/j.biopha.2018.11.123 13.
-
7. Blaszczyk N, Rosiak A, Kaluzna-Czaplinska J. The
potential role of cinnamon in human health. Forests. 2021;12(5):1-17. doi:10.3390/f12050648
-
8. Kadar DAN, Tikus S sgpt H, Diinduksi Y, Januari
-
D, Mei D. Pengaruh Ekstrak Kayu Manis Terhadap 14. Gambaran Histopatologi Dan Kadar Sgot-Sgpt Hepar Tikus Yang Diinduksi Parasetamol. Life Sci. 2016;4(1):29-37.
-
9. Eidi A, Mortazavi P, Bazargan M, Zaringhalam J.
Hepatoprotective activity of cinnamon ethanolic 15. extract against CCL 4-induced liver injury in rats.
EXCLI J. 2012;11:495-507. doi:10.17877/DE290R-4957 16.
-
10. Rasydy LOA, Supriyanta J, Novita D. Formulasi
Ekstrak Etanol 96% Daun Sirih Hijau (Piper Betle
-
L.) Dalam Bedak Tabur Anti Jerawat Dan Uji 17. Aktivitas Antiacne Terhadap Staphylococcus Aureus. J Farmagazine. 2019;6(2):18.
doi:10.47653/farm.v6i2.142
-
11. Knodell R, Ishak K, Black W, et al. Formulation
and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. J Hepatol. 2003;38:382-386. doi:10.1016/S
Wang P, Pradhan K, Zhong X bo, Ma X. Isoniazid metabolism and hepatotoxicity. Acta Pharm Sin B. 2016;6(5):384-392. doi:10.1016/j.apsb.2016.07.014 Rahayu L, Yantih N, Supomo Y. Analisis SGPT dan SGOT pada Tikus yang Diinduksi Isoniazid untuk Penentuan Dosis dan Karakteristik Hepatoprotektif Air Buah Nanas (Ananas comosus L. Merr) Mentah. J Ilmu Kefarmasian Indones. 2018;16(1):100-106. Antasionasti I, I J. Aktivitas Antioksidan Ekstrak Etanol Kayu Manis (Cinnamomum burmani) Secara In Vitro / Antioxidant Activities Of Cinnamon (Cinnamomum burmani) In Vitro. J Farm Udayana. 2021;10(1):38. doi:10.24843/jfu.2021.v10.i01.p05 Hanifa DD, Hendriani R. Tanaman herbal yang memiliki aktivitas hepatoprotektor. Farmaka. 2016;14(4):43-51.
Redha A. Flavonoid: Struktur, Sifat Antioksidatif dan Peranannya Dalam Sistem Biologis. J Berlin. 2010;9(2):196-202. doi:10.1186/2110-5820-1-7 Lagarde F, Beausoleil C, Belcher SM, et al. Nonmonotonic dose-response relationships and endocrine disruptors: A qualitative method of assessment -No section-. Environ Heal A Glob Access Sci Source. 2015;14(1):1-15.
doi:10.1186/1476-069X-14-13

http://ojs.unud.ac.id/index.php/eum
doi:10.24843.MU.2024.V13.i02.P02
Page 10
Discussion and feedback