ACUTE PAIN MANAGEMENT IN CATS: ASSURANCE OF FELINE WELFARE FULFILLMENT WITHIN CLINICAL SETTING
on
Buletin Veteriner Udayana Volume 15 No. 5: 930-942
pISSN: 2085-2495; eISSN: 2477-2712 Oktober 2023
Online pada: http://ojs.unud.ac.id/index.php/buletinvet https://doi.org/10.24843/bulvet.2023.v15.i05.p29
Terakreditasi Nasional Sinta 4, berdasarkan Keputusan Direktur Jenderal Pendidikan Tinggi, Riset, dan Teknologi No. 158/E/KPT/2021
Acute Pain Management in Cats: Assurance of Feline Welfare Fulfillment Within Clinical Setting
Michael Gunawan1*, Nindya Dwi Utami1, Deni Noviana2
-
1My Vets Animal Clinic, Jl. Kemang Selatan VIII No.7A, RW.7, Cipete Sel., Kec. Cilandak, Kota Jakarta Selatan, Daerah Khusus Ibukota Jakarta 12730;
-
2School of Veterinary Medicine and Biomedical Sciences, IPB University, Jl. Agatis Kampus IPB Dramaga, Bogor, West Java, Indonesia, 16680.
*Corresponding author email: michgun68@gmail.com
Abstract
Assessing, acknowledging, and managing pain in feline patients are often challenging. Failure to recognize and protect the cats from the harmful effects of nociception, therefore, often ensues. Feline patients that are afflicted with illness and tissue trauma that may arise from trauma or surgery have acute pain that needs to be addressed. Acute pain can present with varying degrees of intensity, a parameter of sensory-discriminatory aspect, and unpleasantness, a perceived feeling that is integral to affective aspect. Notwithstanding its usefulness to protect the tissue from further damage, acute pain is detrimental to the feline’s welfare, comprising physical and mental wellness if not managed properly. Uncontrolled pain has also been associated with increased morbidity, prolonged hospitalization period due to delayed recovery, increased health-care cost, and decreased quality of life. This review article is made with the aim to elucidate how acute pain in cats is recognized, prevented, and treated, both pharmacologically and non-pharmacologically.
Keywords: Acute pain; feline welfare; nociception; trauma.
This article was presented at the Animal Welfare Indonesia 1st Conference 2022 seminar organized by JAAN supported by FOUR PAWS on December 14 2022, and has passed selection and review by the seminar editorial team. Buletin Veteriner Udayana published this article under a cooperation contract between Udayana University and JAAN.
INTRODUCTION
Pain in cats is often overlooked. The inability of animals in general to communicate their pain is natural, but it does not nullify the likelihood that they do indeed experience pain. Studies have found that consequences like increased morbidity, impaired quality of life, hampered recovery and subsequent increase in hospitalization period and health-care cost entail refractory pain (Gan, 2017). The lack of pain assessment, recognition, and management has been reported, especially among older and male practitioners, and those who practice in smaller clinical settings, with multifactorial causes such as limited access to various analgesics, concern over opioid’s adverse effects, inadequate curriculum material about pain management taught in
veterinary schools and differing subjective perception of pain between the sexes (Dohoo and Dohoo, 1996; Hugonnard et al., 2004; Joubert, 2001). Furthermore, validated pain assessment tools were not widely used within the clinical setting (Coleman and Slingsby, 2007). When pain is unrecognized, less analgesic is used, leading to inadequate pain management (Simon et al., 2017).
According to the International Society of Feline Medicine, the employment of pain scales that are not approved for use in cats was a hurdle that recently has gotten a breakthrough (Steagall et al., 2022). Admittedly, there are species-related pain-related alterations that become major limitations, especially when using dog-validated pain assessment tools to assess pain in cats. These differences can also be
seen within the species, based on the pain threshold, temperament, and different cause of pain each individual can have (Reid et al., 2018; Steagall et al., 2022). There are now three validated feline-specific pain assessment tools that can be employed, although the use of whichever will need training for the veterinary staff i.e. the clinicians and nurses (Belli et al., 2021; Evangelista et al., 2019; Reid et al., 2017). The use of these validated pain scales has also led to an advancement in research and clinical studies, leading to discovery of feline-specific analgesics that significantly improve the margin of safety of the administered drugs (Steagall et al., 2022).
RESEARCH METHOD
This review article will try to elucidate the mechanism behind pain perception, the use of these advent feline-specific pain assessment tools, and the art of feline acute pain management, both pharmacologically and non-pharmacologically.
RESULTS AND DISCUSSION
Acute Pain: Why and How It Happens
Acute pain arises from any insults that result in tissue injury and subsequent inflammation. Acute inflammation is a physiological response to tissue injury and death to facilitate tissue recovery. For instance, inflammation gives rise to prostaglandin, a vasoactive, pyrogenic, and neuroactive eicosanoid that facilitates not only the amplification of inflammation, but also inflammation resolution, perception of pain to protect the affected area from further damage through use withdrawal (eg., animals with painful limbs
instinctively will not perambulate as much to protect the diseased limbs), and prevention in the proliferation of potentially pathogenic microorganisms through an increase in body or the affected area’s temperature (Ackermann, 2017). This is termed as “protective pain”. Despite its beneficial purposes, uncontrolled acute pain does more harm than good, because it
is associated with perpetual tissue deterioration and exacerbation of the preexisting tissue damage. Furthermore, pain that exceeds the threshold of the individual will result in, but not limited to, reduced quality of life (eg., decreased sleep quality, emotional instability due to the perceived “ceaseless perceived pain”), stress that leads to immunosuppression, hypertension, and behavioral changes that can affect the human-animal bond (Muir, 2015). Hence, acute pain should be managed, not necessarily to eliminate its “protective” role of it, but to control the pain so that it does not exceed the “just enough” level needed for protection. Furthermore, uncontrolled pain can result in pain that is unnecessary and results in increased pain perception that goes beyond its adaptive, protective, and recuperative purpose, often referred to as maladaptive pain (Costigan et al., 2009).
Pain is initiated by peripheral stimuli with sufficient intensity that are detected by nociceptors. These stimuli are often referred to as noxious stimuli, and those that fall in this category have the likelihood to injure the body tissue. Nociceptors have some differences in their axons: Aδ and C fiber axons. Aδ fiber is lightly myelinated and has small diameter, transforming mechanical and thermal stimuli into electrical signals and conducting them more slowly, and giving rise to sharp, rapid pain that is responsible for withdrawal reflex in acute pain. C fibers are unmyelinated and have even smaller diameters than Aδ. They respond to chemical, thermal, and mechanical stimuli, and conduct more slowly than the Aδ, responsible for the dull pain a few moments after the first noxious insult is made (Fields et al., 1998). These electrical signals then get transmitted to the central nervous system through the dorsal horn ganglia. This signal is then processed, resulting in increased alertness and altered behavioral strategies that are used to avoid further contact with the harmful stimuli (Latremoliere and Woolf, 2009). Noxious stimulus with such intensity that
overwhelms the pain threshold results in dysfunction of the components of either peripheral or central nervous system, or both. Though not necessarily a pathologic state, which instead could become integral parts of protective pain, allodynia (ie., pain that results from normally unpainful stimuli) and hyperalgesia (ie., increased pain sensitivity) can arise. Increased neuronal membrane’s excitability and synaptic transmission, or reduced neuronal inhibition, can be the causes of such states. This phenomenon is termed as functional plasticity. When the nociceptive afferent neurons are deluged with increased noxious inputs, functional plasticity of the afferent nociceptive neurons occurs, altering the receptive field surface area and threshold. During this plastic state, sensitized peripheral nociceptors become more excitable, so more electrical signals are conveyed towards the central nervous system, evoking a heightened state of pain in affected and the surrounding areas (ie., primary and secondary hyperalgesia, respectively), even with stimuli that are not commonly painful (ie., allodynia) (Tsagareli, 2019).
Assessing Acute Pain in Cats
Effective pain management is only possible if valid, reliable and sensitive pain assessment tool is readily accessible to be utilized. A pain assessment tool is considered reliable and sensitive if it has scientific proofs of consistent results reproducibility in distinction of painful animals from the healthy ones, valid if it is proven to be able to assess the targeted parameters. These features are essential because pain assessment tool is going to be needed for constant reassessments to evaluate whether the interventional analgesic given is adequate to relieve the pain of the affected individual. The assessment result will not give valid and accurate result if it could not discriminate between painful and non-painful individuals, and between different pain intensities (Calvo et al., 2014; Steagall and Monteiro, 2019).
Pain is multidimensional. In human medicine, Melzack and Casey (1968) described pain in three dimensions: sensory-discriminative, affective-motivational, and cognitive-evaluative. The sensory-discriminative aspect of pain is assessed through a thorough physical examination to determine pain location and intensity, and obtainment of complete medical history to determine the duration of the pain. This domain is usually assessed concurrently with the second aspect, affective-motivational, which explains the demeanor and behavioral changes that arise from the extent the pain can be perceived as unpleasant to the affected cat. Straightforward changes in the demeanor such as mental dullness or aggression, or more subtle changes like different head position and whiskers tensity, can be picked up from the initial assessment, with the help of pain assessment tool and hands-on physical examination. The third domain, cognitive-evaluative, is virtually implausible to evaluate because it involves cognitive alterations due to experienced pain (Merola and Mills, 2016a; Steagall and Monteiro, 2019).
There are common pitfalls that can make pain evaluation ambivalent, especially when relying solely on behavioral or physiological changes. First, detection of these changes can be subjective, as many studies have pointed out (Dohoo and Dohoo, 1996; Hugonnard et al., 2004; Joubert, 2001; Williams et al., 2005). This is exacerbated by the lack of use of validated pain assessment tools (Coleman and Slingsby, 2007). Variations in pain behaviors between individuals have complex confounding factors that can arise from the environment, the individual’s disposition, and anesthetic influence (in post-operative patients). The latter is especially true in post-operative patients, where veterinarians would frequently discharge the animals following elective surgical procedure (eg., orchiectomy) without analgesics, because there is a bias of dysmorphia following anesthesia
recovery (Buisman et al., 2016; Williams et al., 2005).
Painful cats can be characterized by the change in their demeanor, either by becoming more dull or aggressive. However, other conditions may cause this change, or overshadow coexisting pain perception, such as patients presenting with shock and central nervous system disorders. Cats, being closely related to other big cats, may conceal painful behaviors due to their predatory instinct (Berteselli et al., 2014). Individuals with high fear, anxiety, and stress levels may have alterations in their physiological and mental variables, especially when they are evaluated in unfamiliar setting, so metrology evaluation scores higher when the cats are assessed at home than in clinical settings. Anorexia is also a sign of acute pain. However, not eating can also mean they are already in satiated condition (Steagall et al., 2022). Although acute pain assessment by the judgment of either behavioral or physiological alterations is insufficient (Merola and Mills, 2016b, 2016a), the display of multiple behavioral signs can be a tip-off information that encourages further pain assessment and recognition. Due to the subjectivity of pain evaluation, pain assessment metrology is used to help make a more sound, unbiased clinical judgment. Three validated feline-specific pain assessments are now available (Belli et al., 2021; Evangelista et al., 2019; Reid et al., 2017). As mentioned before, pain is multidimensional. Therefore, a good, validated pain assessment should be composite, comprising sensory and other aspects that reliably detect the feeling of unpleasantness due to pain. These three pain assessment tools have their respective discerned parameters, and cut-off value of which interventional analgesic is indicated once reached or even surpassed. Nonetheless, none of these tools have a sensitivity or specificity of nearly 100%, making these, to some extent, still possible to overlook pain in cats. Whatever metrology is used, sufficient training is
necessary to reduce bias and judgment differences among the veterinary staff. There are three pain assessment scale described in this article.
Universidade Estadual Paulista (UNESP)-Botucatu (UFEPS) multidimensional pain assessment scale
The initial study of the use of this pain scale was conducted in cats with postoperative pain, following ovariohysterectomy. However, many studies have used this pain scale. It discerns pain expression (miscellaneous behaviors, vocalization, reaction to palpation of surgical wound), psychomotor change (level of activity, posture, attitude), and physiological variables (appetite, blood pressure) (Steagall and Monteiro, 2019). This scale is also the first validated multilanguage feline-specific assessment tool. UNESP-Botucatu scale, however, has limited practicability due to its extensive subscales and assessed variables, including blood pressure monitoring. This issue has been addressed by the creation of a shorter version (UFEPS-Short Form [SF]), evaluating the cat’s posture, level of activity, attitude, and reaction to palpation on painful area. A study conducted by Belli et al (2021) indicated good validity, although the current study lacked a more randomized, blinded evaluation. It has strong criterion correlations between the UFEPS and UFEPS-SF. Both UFEPS and UFEPS-SF have moderate sensitivity with a known increase in psychomotor change score in cats previously induced with ketamine (Buisman et al., 2016). Both UFEPS and UFEPS-SF are slightly higher in specificity than sensitivity. Inter-rater reliability remains as a limitation for this pain scale. The original study performed by Brondani et al. (2013) and the most recent study where veterinary anesthesia and analgesia specialists were enrolled to assess (Luna et al., 2022) indicated higher interrater reliability (CI 0.93-0.97, and CI 0.84 respectively), whereas other studies reported lower, moderate inter-rater reliability (CI 0.79-0.82) (Belli et al.,
2021), with considerably high variability in one study (Benito et al., 2017). It is recommended to access the site (www.animalpain.com.br) for online training, using the provided videos and other information. The cut-off value of UFEPS-SF and UFEPS are ≥ 4/12 and ≥ 7/24, respectively.
Glasgow composite measure pain scaleFeline (Glasgow CMPS-F)
Glasgow CMPS-F included behavioral parameters like vocalization, level of activity, posture, attention to wound, response to palpation, and reaction in response to the caretaker. The most updated version also included facial expressions of pain (shape of muzzle, ear pinna position). The developmental stage of this tool creation included positive control groups with different health problems that result in pain: trauma, surgery, and other medical illnesses (Calvo et al., 2014). Hence, it can be more appropriate to use in clinical settings where diverse pain-inciting agents may present. Moreover, as opposed to the validity assessment of UFEPS, Glasgow CMPS-F validation was conducted in clinical environments where it was assessed by observers of different levels of experience, so it can be more applicable in small animal practices where veterinary anesthesia and analgesia specialists are not present. This study also proved a much higher pain discriminatory ability among the observers, resulting in less inter-rater reliability (Reid et al., 2018). Nonetheless, this pain scale needs more studies to prove its reliability. Rescue/interventional analgesia is given when the cut-off point of ≥ 5/20 is reached (Gruen et al., 2022; Steagall and Monteiro, 2019).
Feline Grimace Scale (FGS)
FGS is the most recent validated pain assessment tool which uses facial components of the cats. Validated grimace scales have been developed for other species, such as mice (Langford et al., 2010), rabbits (Keating et al., 2012), and horses (Dalla Costa et al., 2014). The
evaluated facial components, referred to as “action units” that construct a facial expression, is composed of ear position, degree of orbital tightening, muzzle tension, whisker position, and head position. This assessment tool can be less invasive (due to the absence of hands-on approach) and more practical. Indeed, the assessment can be carried out in real-time or through a photograph of the cat, unperturbed and unrestrained inside the kennel, as any forms of handling and restraints will affect the facial expression (Holden et al., 2014). FGS has been reported to be valid, reliable, sensitive, and specific. However, note should be taken that brachycephalic breeds may present with either false positive or false negative results (ie., higher and lower score than the cut-off point, respectively), due to their different facial conformities compared to mesocephalic breeds (Evangelista et al., 2019). The cut-off point for FGS is ≥ 4/10. Online training is provided on its website (www.felinegrimacescale.com).
Pain Management: A Pre-emptive, Multimodal Approach
From previous paragraphs, the author beseeches the readers that becoming sufficient in pain assessment is just as important as becoming proficient in physiological evaluation. Failure to recognize pain results in oligoanalgesia, a state of inadequacy in pain management (Simon et al., 2017). When referring to pain management, the term analgesia is conjured up. However, the term itself means “the absence of pain sensation”, which may become an unfeasible objective to obtain when managing pain. Pain should be effectively controlled, to the point that it does not interfere with the welfare of the patient. Conversely, when pain is expected, as in surgery, it is now generally accepted that pain prevention is favorable than once tissue damage and subsequent pain are produced (Gruen et al., 2022). Pain management goal should focus on the most pain alleviation and comfort level that can be achieved, while minimizing the potential
adverse drug reactions. Therefore, a multimodal approach is advised to: (1) control pain through multiple drugs with differing mechanism of actions, as pain pathways are often redundant (Zochodne, 2012), and (2) reduce the drug dose and/or administration frequency when multiple analgesics are used. The authors will try to summarize some of pharmacological and non-pharmacological interventions that are proven to provide analgesia in cats. Nevertheless, some surgical procedures, or pathomechanisms, may elicit more pain than the others, and hence could become more challenging to manage.
Pharmacologic Agents
There are a few things to be considered in the administration of analgesics. Steagall et al (2022) proposed the acronym “T.E.L.L.S” to determine the type, dose, frequency, and route of analgesic(s) to be given to the patient: Type of noxious stimuli (eg., visceral, neuropathic,
oncologic, somatic, orofacial), Expected duration of the nociception (eg., transient stimuli such as endoscopy vs extended pain perception as in surgery), Location of noxious stimuli (eg., onychectomy procedure can benefit from femoral and sciatic nerve blocks), Location of the patient throughout the medication (out- vs inpatient), and Severity of noxious stimuli.
Opioids
Opioids act on opioid receptors, and opioids can be categorized based on the matching receptor: mu, delta, and kappa. Although the functional effects of each receptor are different once activated, they have similar cellular responses after becoming activated. Dissociation of alphaguanosine triphosphate (aGTP) and betagamma complex of the receptor’s plasma membrane results in decrease in intracellular adenylyl cyclase, hence in turn reducing cyclic adenosine monophosphate levels, dampening signal transduction. Moreover, activation of mu-opioid receptors increases potassium and decreases calcium influx, rendering the
neurons hyperpolarized. Since the central nervous system expresses an ample of opioid receptors, less pain is perceived once these receptors are activated (Pathan and Williams, 2012).
Because opioid receptors are expressed in high levels in the nervous system, according to a recent proposal for acute pain management in both cats and dogs, opioids should be the first choice when pain is evident, but the causative factor is not determinable immediately. Once the pain has subsided into a more comfortable level, diagnostic workup should be performed to pinpoint the etiology of the pain (Gruen et al., 2022).
Opioid selection should be based on the result of veterinary staff’s pain assessment of the patient. If moderate to severe pain (eg., cat with pancreatitis, cat who undertakes invasive surgery like ovariohysterectomy and orthopedic procedures) is indicated or predicted, opioid that exerts more activation of mu-opioid receptor is recommended. Single dose of methadone (0.2-0.6 mg/kg IV/IM q6h) or pethidine (5-10 mg/kg SC/IM q1-2h) can be given initially, and reassessment should be done to evaluate the requirement of other rescue analgesia. In the authors’ experience, methadone is unavailable in our practice, so pethidine is the only full mu-opioid we can get access to. However, pethidine has short plasma half-life, and repeated injection can add stress and exacerbate pain. If pain is indicated to be prolonged, constant rate infusion (CRI) of fentanyl (0.005 mg/kg bolus, followed by 0.003-0.02 mg/kg/h IV) is recommended. Although there was a concern of the use of morphine due to its need for glucuronidation (Bell, 2009), it has been reported that there is no significant difference in morphine elimination rate between cats and dogs. Instead, cats may have reduced active metabolite bioavailability (Bloomfield et al., 2001). Notwithstanding, morphine still remains as the archetypal analgesic that is proven to be effective for acute moderate-to-severe pain
management in cats (0.1-0.2 mg/kg IV/IM q6h, 0.1-0.2 mg/kg epidurally q24h) (Steagall et al., 2022). Mild to moderate pain can be managed with buprenorphine (0.02-0.04 mg/kg IV/IM q8h), a partial mu-opioid receptor agonist. Nonetheless, variabilities in the response to the opioid treatments, irrespective of the receptors they are working on, have been reported, so it is important to always reassess the patient to cater to the patient’s need on individual basis (Bloomfield et al., 2001; Johnson et al., 2007).
Although opioid use is recommended in management of acute pain, the authors acknowledge that opioids may be inaccessible in many practices. This shortage has become an issue in the effectiveness of a pain management protocol when opioid is not included, as NSAID alone has been proven to be inadequate to control postovariohysterectomy pain, even when intraperitoneal bupivacaine is added (Steagall et al., 2018). The involvement of multiple stakeholders like governmental bodies, pharmaceuticals, and drug regulatory bodies may be necessary to resolve this inaccessibility to opioid drugs, especially to address the state of oligoanalgesia in cats, and other animals (Steagall et al., 2022).
Non-steroidal anti-inflammatory drugs (NSAIDs)
As the name suggests, NSAID works best when acute tissue damage and subsequent inflammation are highly determinable. Oftentimes, NSAID can be indicated in conditions such as neoplasia, lymphoplasmacytic gingivostomatitis, idiopathic cystitis, uveitis, and even in other conditions where short-term use of NSAID can be opted to make a better clinical judgment based on the therapeutic response, owing to its anti-inflammatory, antipyretic, and analgesic properties. In cats, there are only two approved NSAIDs for use in cats. NSAIDs exert its antiinflammatory, antipyretic, and analgesic actions by inhibiting cyclooxygenase
(COX), an enzyme that catalyzes the production of prostanoids. There are two isoenzymes: COX-1 and COX-2. Concerns regarding the use of non-selective NSAID raise from its inhibitory effect on COX-I, which is constitutively expressed in nearly all cells in the body to carry out physiological functions, including maintenance of renal perfusion and electrolyte homeostasis, and gastric mucosal integrity (Fromm, 1987; Kim, 2008). Meloxicam (0.1 mg/kg q24h PO initially, then 0.05 mg/kg PO; 0.2 mg/kg IM/IV single injection, followed by oral meloxicam the days onwards) and robenacoxib (1 mg/kg q24h PO, up to 6 days; 2 mg/kg q24h SC, up to 3 days) are the only two approved NSAIDs for use in cats. Studies have indicated generally accepted use safety in cats, even when used for extended period (dose 0.01-0.03 mg/kg q24h per oral), in which cases can benefit the cats by reducing pain level, especially in chronic musculoskeletal pain (Gunew et al., 2008). Meloxicam is also present as a promising analgesic in cats with “stable” chronic kidney disease (ie., cats with CKD of IRIS stage 1-2, with minimal to no change in body weight and plasma creatinine level in 1-2 months), although this study was retrospective, therefore is subject to biases (B. Monteiro et al., 2019). Robenacoxib is licensed for therapeutic use of up to 6 days. Although pharmacokinetic studies have shown robenacoxib to have elimination half-life of 2 hours in cats, the high protein-binding rate may facilitate persistence of robenacoxib within inflamed tissue. It should be underscored that cats have different hepatic metabolism in comparison with dogs, and studies have reported inter-individual differences in the rate of NSAID elimination, hence different toxicity thresholds and responses to analgesic, antipyretic and antiinflammatory properties among feline individuals when NSAID is administered, even if approved NSAID is used, are not uncommon (Court, 2013; Sparkes et al., 2010).
Locoregional Anesthetics
Local anesthetic should also become a mainstay of a multimodal acute pain management plan. Local block has been shown to reduce the required dose of opioid to prevent and ameliorate post-surgical acute pain (Cicirelli et al., 2022). Local anesthetics work by preventing pain impulse from reaching the central nervous system. For example, lidocaine (dog: 4-6 mg/kg; cat: 2-4 mg/kg) and bupivacaine (dog: 1-2 mg/kg; cat: 1 mg/kg) work by inhibiting voltage-gated sodium channels, thereby preventing nociceptors
depolarization and propagation of action potential and, consequently, propagation and conduction of pain stimulus (Yang et al., 2020). In surgery, locoregional block protocol stabilizes nociceptive indicators like heart and respiratory rate, and reduces pain score when compared to the patients who only receive general anesthetics and systemic analgesics.
As the name suggests, drugs under this category are used for its locoregional “numbing” effect. Inadvertent rapid
intravenous injection can result in
deleterious adverse effects, as its
pharmacodynamic property will interrupt a more systemic neuronal modulation, transmission, and propagation. Side effects after accidental intravascular injection include, but not limited to, muscle twitching and/or fasciculation, weakness, and cerebral disturbances like excitation and seizure. It is, therefore, advised to aspirate first before performing a logoregional block. Rare adverse effects, but have been reported from time to time, include methemoglobinemia due to benzocaine administration, anaphylactic reaction (reported to have increased incidence if ester forms are formed like procaine and benzocaine, and if the local anesthetic drug used contains
methylparaben) (Grubb and Lobprise, 2020).
It should be underscored that local anesthetic drugs work on dose- and concentration-dependent manners, with its
therapeutic action exerted through inhibition of nociceptive impulse transmissions. However, because
peripheral nerves contain both sensory and motor neurons, motor blockade should always be anticipated. This motor blockade may be useful in instances where ambulatory restriction is favored, especially in cases of musculoskeletal trauma and/or surgery.
Adjunctive Analgesics
Although NSAIDs and opioids can be used concurrently as a multimodal approach, oftentimes pain management requires other pharmacologic agents to provide breakthrough in pain pathway redundancy. It is helpful to remember the T.E.L.L.S. that has been mentioned previously. For instance, if neuropathic pain is indicated, the use of gabapentin (alpha-2-delta calcium channel disruptor), or the addition of ketamine (N-methyl-D-aspartate [NMDA] antagonist) can be considered. Gabapentin (10 mg/kg q8-12h per os) exerts its neuropathic painmodulating property through disruption of alpha-2-delta voltage-gated calcium channel (VGCC), which reduces interactions between its subunits with NMDA receptors. Both VGCC and NMDA receptors are found in the central nervous system, modulating pain sensory signals, and the inhibitory effect of gabapentin therefore will result in decreased pain sensation, though gabapentin use alone without other different analgesics may not alleviate acute pain (Dolphin, 2013; Park and Luo, 2010; Steagall et al., 2022). Although ketamine is extensively excreted through renal elimination route and may accumulate in the plasma in patients with renal insufficiency, it has been indicated that sub-anesthetic dose of ketamine administered continuously (ie., CRI, 0.01 mg/kg/minute) is safe and can provide neuropathic pain amelioration through downregulation and reduced
phosphorylation of NMDA receptors (Steagall et al., 2022).
Other Supportive Pharmacologic Agents
Besides from provision of analgesia, patients with other existing health conditions should also be managed properly. For example, nauseous and vomiting cats should be controlled through the administration of antiemetic agent (eg., maropitant 1 mg/kg q24h IV; ondansetron 0.5 mg/kg IV bolus, followed by 0.5 mg/kg/h CRI for 6 hours) and/or gastrointestinal protective agents (decision to use such agents should consult a consensus statement regarding the rationale for gastrointestinal protectants administration [Marks et al., 2018]). Cats with high fear and anxiety level may benefit from gabapentin (20 mg/kg, 90 to 120 minutes before anxiety-inducing event is predicted). Alprazolam (0.125-0.25 mg per cat, per oral), a benzodiazepine agent, can also be used pre-clinic visitation (Rodan et al., 2011). The use of premedication like medetomidine (alpha-2 agonist, 0.04-0.08 mg/kg IM) provides a degree of muscle relaxation, sedation, and analgesia, though patients with compromised cardiovascular health are contraindicated. Tramadol (1-4 mg/kg q12h per oral), a racemic mixture that possesses opioid receptor agonist, serotonin and norepinephrine reuptake inhibitory properties, is also a promising analgesic in cats, providing pain relief in dose-dependent manner, and has been proven to be efficacious in lessening the intensity of osteoarthritic pain in cats (B. P. Monteiro et al., 2017; Pypendop et al., 2009). Notwithstanding its antinociceptive property, its use in cats is limited, mainly due to the feline’s sensitive sense of taste (Steagall et al., 2022).
Non-Pharmacological Interventions
A positive interaction between the caretaker and the cat is the hallmark of nonpainful cats. Cats should be approached and handled gently to maximize the level of comfort and prevent exacerbation of pain. Cage enrichment is recommended. For instance, provision of a card box for hiding and perching, and layers of sheets for comfortable bedding are encouraged. The
ward environment should be kept as quiet as possible to prevent stress in cats (Steagall and Monteiro, 2019). Performing physical examination and medical procedures should be done from those that are less stressful and/or invasive. When administering refrigerated pharmacologic agents, lowering the temperature into room temperature is recommended. Although easier said than done, handling and restraining of the cat should preserve the cat’s natural posture as much as possible (Rodan et al., 2011)
As a sensitive being, cat’s disposition is reinforced at young age through the experience from its surrounding. Client education about the importance of things like positive behavior conditioning through rewarding system, introduction to felinefriendly handling, and introduction to car rides also plays an integral part in the fulfillment of feline welfare. Throughout the examination, cat should be kept as calm as possible, and the use of gabapentin 90120 minutes prior to consultation (consult the pharmacologic agents section of this article) is recommended in stressful individual (Rodan et al., 2011).
Other adjunct therapies are emerging and claimed to be as useful as their pharmacologic counterparts. For example, laser therapy (photobiomodulation) has gained popularity because it is claimed to abate pain sensation in cases of arthritis, fracture and post-surgical healing, and in more feline-specific disorders like hyperesthesia syndrome, idiopathic cystitis, and atopic syndrome with eosinophilic granuloma complex (Lavallee and Olson, 2017). A blinded study in cats following ovariohysterectomy indicated a decrease in rescue analgesia requirement in cats receiving laser acupuncture at the ST36 and SP6 acupoints, compared to the group that only received rescue analgesia after ovariohysterectomy (Marques et al., 2015). Cold therapy, one of the oldest non-pharmacological interventions of which use was dated as far back in 1812, is also proven to be beneficial in post-surgical acute pain
relief. Cold modulates pain perception by, but not limited to, decreasing nociceptors activation, thwarting conduction velocity along the peripheral axons, promotion of vasoconstriction to reduce oedema and the subsequent pain-associated mediators, and decreasing secondary hypoxic damage via metabolism deceleration. A review written by Wright et al (2020) provided practical implementations of cold therapy in many instances of acute pain scenarios in small animals, including cats and dogs.
CONCLUSION
Effective pain control in cats is a serious matter to be reckoned with. Appropriate veterinary staff training for pain recognition and assessment is pivotal in a successful pain management, and all staff, including veterinarians and paramedics, should be involved. Pain control protocol should always be tailored in a case-by-case manner, and the provision of a felinefriendly environment is as important as the administration of pharmacological interventions.
REFERENCES
Ackermann MR. 2017. Inflammation and Healing. Pathologic Basis of Veterinary Disease (Sixth Edition), Mosby. Pp. 73-131.
Bell A. 2009. Constant-rate infusions: part two. Vet Times.
Belli M, de Oliveira AR, de Lima MT, Trindade PHE, Steagall PV, Luna SPL. 2021. Clinical validation of the short and long UNESP-Botucatu scales for feline pain assessment. PeerJ. 9: e11225.
Benito J, Monteiro BP, Beauchamp G, Lascelles BDX, Steagall PV. 2017. Evaluation of interobserver agreement for postoperative pain and sedation assessment in cats. J. Am. Vet. Medical Assoc. 251(5): 544–551.
Berteselli GV, Spiezio C, Normando S, de Mori B, Zaborra CA. 2014. What do domestic cats have in common with
European wildcats? J. Vet. Behav.: Clin. Appl. Res. 9(6): e2.
Bloomfield M, Waters C, Dixon M, Taylor P, Robertson S, Ruprah M, Sear JW. 2001. Morphine, pethidine and buprenorphine disposition in the cat. J. Vet. Pharmacol. Therap. 24(6): 391–398.
Brondani JT, Luna SPL, Minto BW, Santos BPR, Beier SL, Matsubara LM, Padovani CR. 2013. Confiabilidade e pontuação mínima relacionada à intervenção analgésica de uma escala multidimensional para avaliação de dor pós-operatória em gatos. Arquivo Bras. Med. Vet. Zoot. 65(1): 153–162.
Buisman M, Wagner MC, Hasiuk MMM, Prebble M, Law L, Pang DSJ. 2016. Effects of ketamine and alfaxalone on application of a feline pain assessment scale. J. Feline Med. Surg. 18(8): 643– 651.
Calvo G, Holden E, Reid J, Scott EM, Firth A, Bell A, Robertson S, Nolan AM. 2014. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J. Small Anim. Prac. 55(12): 622–629.
Cicirelli V, Burgio M, Lacalandra GM, Aiudi GG. 2022. Local and regional anaesthetic techniques in canine ovariectomy: A review of the literature and technique description. Animals 12(15): ani12151920.
Coleman DL, Slingsby LS. 2007.
Attitudes of veterinary nurses to the assessment of pain and the use of pain scales. Vet. Rec. 160(16): 541–544.
Costigan M, Scholz J, Woolf CJ. 2009. Neuropathic pain: A maladaptive
response of the nervous system to damage. Ann. Rev. Neurosci. 32: 1–32.
Court MH. 2013. Feline drug metabolism and disposition: pharmacokinetic
evidence for species differences and molecular mechanisms. Vet. Clin. North Am. Small Anim. Prac. 43(5): 1039–1054.
Dalla CE, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. 2014. Development of the horse grimace scale (HGS) as a pain assessment tool in horses undergoing routine castration. Plos One. 9(3): e92281.
Dohoo SE, Dohoo IR. 1996. Factors influencing the postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can. Vet. J. 37(9): 552.
Dolphin AC. 2013. The α2δ subunits of voltage-gated calcium channels. Biochimica et Biophysica Acta (BBA) – Biomembranes. 1828(7): 1541–
1549.
Evangelista MC, Watanabe R, Leung VSY, Monteiro BP, O’Toole E, Pang DSJ, Steagall PV. 2019. Facial expressions of pain in cats: the development and validation of a Feline Grimace Scale. Sci. Rep. 9(1): 1–11.
Fields HL, Rowbotham M, Baron R. 1998. Postherpetic neuralgia: Irritable
nociceptors and deafferentation.
Neurobiol. Dis. 5(4): 209–227.
Fromm D. 1987. How do non-steroidal anti-inflammatory drugs affect gastric mucosal defenses? Clin. Investig. Med. 10(3): 251–258.
Gan TJ. 2017. Poorly controlled postoperative pain: prevalence,
consequences, and prevention. J. Pain Res. 10: 2287.
Grubb T, Lobprise H. 2020. Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Vet. Med. Sci. 6(2): 209.
Gruen ME, Lascelles BDX, Colleran E, Gottlieb A, Johnson J, Lotsikas P, Marcellin-Littl, D., and Wright, B. 2022. 2022 AAHA Pain management guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 58(2): 55–76.
Gunew MN, Menrath VH, Marshall RD. 2008. Long-term safety, efficacy and palatability of oral meloxicam at 0.010.03 mg/kg for treatment of osteoarthritic pain in cats. J. Feline Med. Surg. 10(3): 235–241.
Holden E, Calvo G, Collins M, Bell A, Reid J, Scott EM, Nolan AM. 2014. Evaluation of facial expression in acute pain in cats. J. Small Anim. Prac. 55(12): 615–621.
Hugonnard M, Leblond A, Keroack S, Cadoré JL, Troncy E. 2004. Attitudes and concerns of French veterinarians towards pain and analgesia in dogs and cats. Vet. Anaesthesia and Analgesia, 31(3): 154–163.
Joubert KE. 2001. The use of analgesic drugs by South African veterinarians. J. South African Vet. Assoc. 72(1): 57– 60.
Keating SCJ, Thomas AA, Flecknell PA, Leach MC. 2012. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PloS One. 7(9): e44437.
Kim GH. 2008. Renal effects of
prostaglandins and cyclooxygenase-2 inhibitors. Electrolytes and Blood
Pressure: E and BP. 6(1): 35.
Langford DJ, Bailey AL, Chanda ML, Clarke SE, Drummond TE, Echols S, Glick S, Ingrao J, Klassen-Ross T, Lacroix-Fralish ML, Matsumiya L, Sorge RE, Sotocinal SG, Tabaka JM, Wong D, van den Maagdenberg AMJM, Ferrari MD, Craig KD, Mogil, JS. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat. Methods. 7(6): 447–449.
Latremoliere A, Woolf CJ. 2009. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The J. Pain : Official J. Am. Pain Soc. 10(9): 895.
Lavallee JO, Olson J. 2017. FelineSpecific Conditions. In R. J. Riegel and J. C. Godbold Jr (Eds.). Laser Therapy Vet. Med.
Photobiomodulation. Pp. 198–211.
Luna SPL, Trindade PHE, Monteiro BP, Crosignani N, della Rocca G, Ruel HLM, Yamashita K, Kronen P, te Tseng C, Teixeira L, Steagall PV.
2022. Multilingual validation of the short form of the Unesp-Botucatu Feline Pain Scale (UFEPS-SF). PeerJ. 10: e13134.
Marks SL, Kook PH, Papich MG, Tolbert MK, Willard MD. 2018. ACVIM consensus statement: Support for rational administration of
gastrointestinal protectants to dogs and cats. J. Vet. Int. Med. 32(6): 1823– 1840.
Marques VI, Cassu RN, Nascimento FF, Tavares RCP, Crociolli GC, Guilhen RC, Nicácio GM. 2015. Laser acupuncture for postoperative pain management in cats. Evidence-Based Compl. Altern. Med. 2015: 653270.
Melzack R, Casey KL. 1968. Sensory, motivational, and central control
determinants of pain: A new
conceptual model. In D. R. Kenshalo (Ed.), The Skin Senses. Pp. 423–443.
Merola I, Mills DS. 2016a. Behavioural signs of pain in cats: an expert consensus. Plos One. 11(2): 0150040.
Merola I, Mills DS. 2016b. Systematic review of the behavioural assessment of pain in cats. J. Feline Med. Surg. 18(2): 60–76.
Monteiro BP, Klinck MP, Moreau M, Guillot M, Steagall PVM, Pelletier JP, Martel-Pelletier J, Gauvin D, del Castillo JRE, Troncy E. 2017. Analgesic efficacy of tramadol in cats with naturally occurring osteoarthritis. Plos One. 12(4): 0175565
Monteiro B, Steagall PVM, Lascelles BDX, Robertson S, Murrell JC, Kronen PW, Wright B, Yamashita K. 2019. Long-term use of non-steroidal anti-inflammatory drugs in cats with chronic kidney disease: from
controversy to optimism. J. Small Anim. Prac. 60(8): 459–462.
Muir WW. 2015. Pain and stress: Stress-induced hyperalgesia and hypoalgesia. In Handbook of Veterinary Pain Management: 3rd Ed. Elsevier Inc. Pp. 42–60.
Park JF, Luo ZD. 2010. Calcium channel functions in pain processing. Channels. 4(6): 510.
Pathan H, Williams J. 2012. Basic opioid pharmacology: an update. British J. Pain. 6(1): 11.
Pypendop BH, Siao KT, Ilkiw JE. 2009. Effects of tramadol hydrochloride on the thermal threshold in cats. Am. J. Vet. Res. 70(12): 1465–1470.
Reid J, Nolan AM, Scott EM. 2018. Measuring pain in dogs and cats using structured behavioural observation. Vet. J. 236: 72–79.
Reid J, Scott EM, Calvo G, Nolan AM. 2017. Definitive glasgow acute pain scale for cats: validation and
intervention level. Vet. Rec. 180(18): 449.
Rodan I, Sundahl E, Carney H, Gagnon AC, Heath S, Landsberg G, Seksel K, Yin S. 2011. AAFP and ISFM felinefriendly handling guidelines. J. Med. Surg 13(5): 364–375.
Simon BT, Scallan EM, Carroll G, Steagall PV. 2017. The lack of
analgesic use (oligoanalgesia) in small animal practice. J. Small Animal Prac. 58(10), 543–554.
Sparkes AH, Helene R, Lascelles BDX, Malik R, Sampietro LR, Robertson S, Scherk M, Taylor P. 2010. Isfm and AAFP consensus guidelines: longterm use of nsaids in cats. J. Feline Med. Surg. 12(7): 521–538.
Steagall PV, Benito J, Monteiro BP, Doodnaught GM, Beauchamp G, Evangelista MC. 2018. Analgesic effects of gabapentin and buprenorphine in cats undergoing ovariohysterectomy using two painscoring systems: a randomized clinical trial. J. Feline Med. Surg. 20(8): 741– 748.
Steagall PV, Monteiro BP. 2019. Acute pain in cats: Recent advances in clinical assessment. J. Feline Med. Surg. 21(1): 25–34.
Steagall PV, Robertson S, Simon B, Warne LN, Shilo-Benjamini Y, Taylor
S. 2022. 2022 ISFM Consensus
Guidelines on the Management of Acute Pain in Cats. J. Feline Med. Surg. 24(1): 4–30.
Tsagareli MG. 2019. Hyperalgesia and allodynia: a closer look. symptoms, mechanisms and treatment. NOVA Science Publishers.
Williams VM, Lascelles BDX, Robson MC. 2005. Current attitudes to, and use of, peri-operative analgesia in dogs and cats by veterinarians in New Zealand. New Zealand Vet. J. 53(3): 193–202.
Wright B, Kronen PW, Lascelles D, Monteiro B, Murrell JC, Robertson S, Steagall PVM, Yamashita K. 2020. Ice therapy: cool, current and
complicated. J. Small Anim. Prac. 61(5): 267–271.
Yang,X, Wei X, Mu Y, Li Q, Liu J. 2020. A review of the mechanism of the central analgesic effect of lidocaine. Medicine. 99(17): e19898.
Zochodne D. 2012. Neuropathic pain: redundant pathways, inadequate therapy. Can. J. Neurol. Sci. 39(4): 409–410.
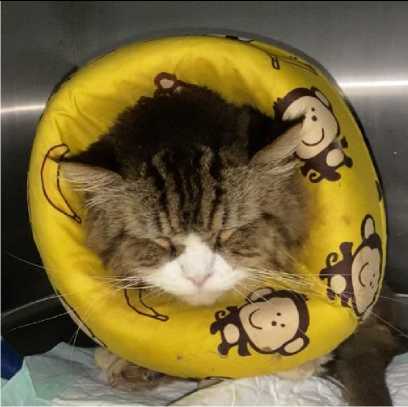

Figure 1. A cat with painful abdomen due to urinary obstruction. This cat was depressed, anorexic, and expressing “grimace” (Self documentation)
Figure 2. Same cat in figure 1, after rescue analgesia was given. Although some of its facial components still indicated pain, the cat was appetent, of which behavior is usually absent in cat with uncontrolled pain (Self documentation)
942
Discussion and feedback