In Vitro And Ex Vitro Propagation Of A Wild-Extinct Fern Lygodium Circinnatum (Burn.F) Sw. Grown In Bali
on
Jurnal Bumi Lestari, Volume 16 No. 2, Agustus 2016, hlm. 131-138
IN VITRO AND EX VITRO PROPAGATION OF A WILD-EXTINCT FERN Lygodium circinnatum (BURN.F) SW. GROWN IN BALI
Rindang Dwiyani*
Faculty of Agriculture, Udayana University, Jalan PB Sudirman, Denpasar, Bali, Indonesia, Post code 80232.
*Email : rindangdwiyani@yahoo.co.id
Abstract
Research concerning of propagation of a wild-extinct fern Lygodium circinnatum had been done at Faculty of Agriculture, Udayana University, Denpasar Bali Indonesia. At some places in Indonesia, as well as Bali, this species is used as materials for making handicraft. In Bali, the species grows wildly in the forest and it is almost extinct due to over gathering. This study aimed to find out method for domestication of L. circinnatum, therefore this wild species can be cultivated, provided materials for making handicraft and might solve the problem of extinction. Various media for growing spores of L. circinnatum in vitro and ex vitro were trialed. In conclusion, full strength of MS media without sugar was the most appropriate media for growth and development of spores of L. circinnatum in vitro. While for ex vitro, the appropriate media were paddy silt-soil and decomposed leaf either with or without addition of foliar fertilizer. However, we suggested ex-vitro cultivation was more appropriate, the technique was much easier and the spores grew faster compared to those of in-vitro.
Keywords: propagation, ex vitro, in vitro, Lygodium circinnatum
Abstrak
Penelitian mengenai perbanyakan spesies paku-pakuan langka Lygodium circinnatum telah dilakukan di Fakultas Pertanian Universitas Udayana, Denpasar, Bali, Indonesia. Di beberapa tempat di Indonesia, seperti halnya Bali, spesies ini digunakan sebagai bahan baku anyaman. Di Bali, spesies ini tumbuh liar di hutan dan hampir punah karena pencarian yang berlebihan. Penelitian ini bertujuan menemukan suatu metode perbanyakan untuk domestikasi L. circinnatum melalui perbanyakan secara in vitro dan ex vitro, sehingga diharapkan dapat mengatasi masalah kelangkaan bahan baku anyaman dari L. circinnatum. Berbagai jenis media untuk perbanyakan secara in vitro dan ex vitro dilakukan dalam penelitian ini. Hasilnya mendapatkan bahwa untuk perbanyakan secara in vitro, media MS (dengan konsentrasi penuh) tanpa gula adalah yang terbaik untuk pertumbuhan dan perkembangan spora secara in vitro. Sedangkan untuk perbanyakan di luar laboratorium (ex vitro), media yang baik untuk pertumbuhan dan perkembangan spora adalah lumpur sawah dan kompos dari daun-daunan, dengan ataupun tanpa penambahan pupuk daun.
Kata kunci: perbanyakan, ex vitro, in vitro, Lygodium circinnatum
In the taxon of plants, a wild climbing fern Lygodium circinnatum is family of Lygodiaceae, class of Polypodiopsida and phylum of Pteridophyta (GBIF Backbone Taxonomy, 2015). The plnat produces spores for its reproduction. Research of genus Lygodium have been done in many aspects by many researchers. For example regarding of antioxidant properties of Lygodium (Jeetendra and Manish, 2011), anthiridiogens identification (Yamauchi et al. 1996), and also about biological control agents (Goolsby et al. 2013), however, there is only few research about the cultivation of Lygodium. In Bali (Indonesia), L. circinnatum grows in the forest, climbs big tree with their creeping shoots. People take the old creeping shoots for making handicraft by lifting the plants from the ground without any replace with new plant, therefore, this species becomes nearly extinct in the forest. This study aimed to find out method for domestication of L. circinnatum, therefore this wild species can be cultivated, provided materials for making handicraft in Bali and might solve the problem of extinction. Previous studies in Indonesia have been done by Raka, et al.(1997) and Rahayu (2006). They cultivated L. circinnatum by splitting young plants from the forest, and then intensively cultivated them by adding fertilizer to stimulate their growth. Unfortunately, those methods of cultivation were failed. We believe this was in accordance with the opinion of Baligar and Duncan (1986), which stated that in the condition of a high amount of nutrient availability, wild plants show slower growth even these nutrients can be toxic to plants. Therefore, we suggested that the earliest life cycle of the plant was required to start cultivate the wild plant. In the recent study, we started to cultivate L. circinnatum from the earliest life-cycle i.e. spores, then we explored methods of cultivation of this species, in-vitro and ex-vitro to find out the appropriate method for cultivation.
Lygodium circinnatum collected by Bali Botanical Garden “Eka Karya” was used as material in the current research. Both In-vitro and ex-vitro experiment were started with collecting spores which are in a structure, called sporangia produced by mature fertile leaves, underside of the leaves. Spore collection of in-vitro method begun with maintaining spores-producing plants. The plants, especially spore-producing leaves, every day for a week were sprayed with fungicide. The leaves then were picked and washed gently, not to damage the sporangium (spores-producing structures at the underside of leaves). Subsequently, the leaves were sprayed with alcohol 95% and put in the laminar. In the laminar, the leaves were air-dried and then put it in a sterile paper envelope and sealed. After that, the paper envelope was kept at room temperature outside laminar. About fourteen days, leaves dried. By lapping the paper envelope with hand, spores will be detached. Furthermore, the paper envelope (that was still closed) was sprayed with alcohol and put in the laminar. Spores then were collected in a sterile container and separated from the leaves. The spore preparation process was done to prevent contamination, because spores are very fine like flour and difficult to be surface-sterilized. Spore collection procedures of ex-vitro method was similar to those performed on in-vitro, just all the work was done outside of the laboratory and did not require prudence to maintain the sterile conditions of the spores. Spore-producing leaves were picked and put in paper envelopes. Furthermore, envelopes (with fertile mature leaves inside) were exposed to the sun, so that the leaves dried. If the leaves have dried, the envelopes were beat on the table or floor so the spores detached. Furthermore, leaves separated from the spores and discarded, while the spores were collected. Figure 1 shows the fertile leaf with sporangia and spores after collecting in the container.
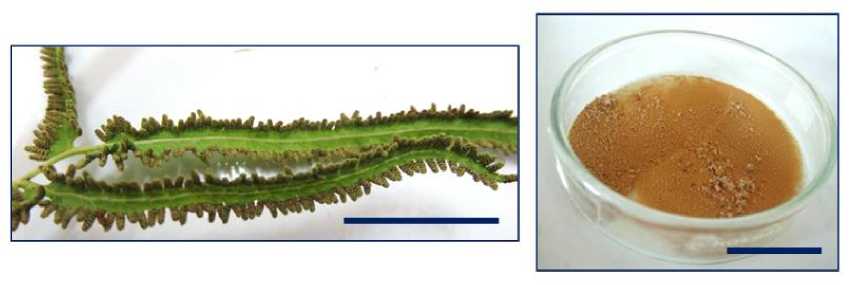
Figure 1. Fertile leaves with sporangia (left) and spores (right). Bar = 5 Cm
Both experiments, in-vitro and ex-vitro were conducted simultaneously during January to July 2014. The in-vitro experiment was done at The Laboratory of tissue culture, Faculty of Agriculture, Udayana University. The experiment was designed as Randomized Block Design. We used six (6) types of medium as treatments, i.e. M1 = Full strength of MS media without sugar; M2 = 1/2 (a half) strength of MS media without sugar; M3 = ¼ (fourth) strength of MS media without sugar; M4 = Full strength of MS media with 20 gL-1 of sugar; M5 = 1/2 (a half) strength of MS media with 20 gL-1 of sugar; M6 = ¼ (fourth) strength of MS media with 20 gL-1 of sugar. Treatments were replicated 5 times. Volume of media was 30 cc per bottle. The media was sterilised with autoclave at 121oC for 30 minutes. About 0.03 g of spores was sown in each treatment. Cultures were maintained in the culture room with temperature of 20oC, and RH of 70%.
Ex-vitro experiment was conducted at a shade house, located at Experimental Station of Faculty of Agriculture Udayana University. Eight (8) types of medium were used as treatments, i.e. T1 = fine grain soil (Indonesian = tanah halus), T2 = paddy-silt soil (Indonesian=lumpur sawah), T3 = decomposed leaves , T4 = moss ( made from root of Asplenium nidus), T5 = fine grain soil added with 1g kg-1 of foliar fertilizer , T6 = paddy-silt
soil added with 1g kg-1 of foliar fertilizer, T7 = decomposed leaves added with 1g kg-1 of foliar fertilizer , and T8 = moss added with 1g kg-1 of foliar fertilizer. Foliar fertilizer used for T5, T6, T7 and T8 contains 20% of total nitrogen (N), 20% of available phosphoric acid (P2O5) and 20% of soluble potash (K2O). Treatments were arranged as Randomized Block Design, replicated 10 times. One and a half (1.5) kilos of each media was put in the plastic pot (top diameter was 15 cm). Spores of about 0.05 g were sown in each media, and then it sprayed with water and was cover with plastic sheet for maintaining high humidity. We also put a plastic tray with water in the bottom of the pot to maintain RH. At the shade house an average daily RH was 80% and temperature was 29.5oC during a period time of experiment.
For both experiments, observation was done for variables of the time of spores germinated, the times of prothalia formation, the time of sporophyte formation, the percentage of prothalia formed, the percentage of sporophytes formed, and height of sporophyte. Prothalium is a heart-shaped structure (seen with microscope) that is formed after spores germinating (Figure 2) Sporophyte is a plant which formed from spore, subsequently after spore germination and prothalium formation.
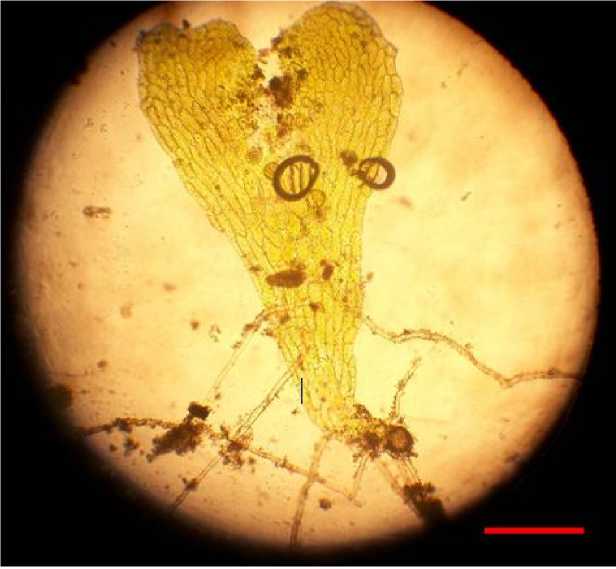
Figure 2. A prothalium (a germinated spore). Bar = 0.5mm
Up to 120 days after spores sowing in-vitro, cultures of M6 treatment were contaminated therefore only 5 treatments were left. The data was not statistically analysed because it was not complete. However, the data of in-vitro treatment was performed at Table 1. Prothalium and sporophyte were not formed on MS medium with sugar, indicating that the sugar inhibited the formation of prothalium, although spores can germinate. Sugar in plant in-vitro culture is a source of energy for the explants to do respiration, subsequently producing energy for the growth of the explant (Saad and Elshahed, 2011), however, it is also a source of energy for the growth of microorganisms as in the case of this study.
MS medium is the most frequently medium used in tissue culture (Saad and Elshahed, 2011),
however, the amount of various ingredients in the medium vary for cultures of different species (Daud et al. 2011). This study found that for culturing spores of L. circinnatum in-vitro, full strength of MS medium resulted in the best growth (Table 1). The result was in line with Abou Dahab et al. (2005) who found that full strength of MS medium was better than ½ strength MS and ¼ strength MS for micropropagation of Ruscus hypoglossum L. Figure 3 shows growth of spores of L. circinnatum at full strength of MS medium without sugar. We suggested that for growing L. circinnatum in-vitro, medium of full strength MS without sugar was the most appropriate.
Table 1. Effects of media on spore germination, prothalus and sporophyte formation in in-vitro Experiment
|
Treatments |
Time of spore germination (das) |
Time of prothalia formation (das) |
Time of Sporophyte formation (das |
|
M1 |
23.70 |
39.70 |
85.04 |
|
M2 |
27.30 |
45.10 |
98.80 |
|
M3 |
49.50 |
65.50 |
110.00 |
|
M4 |
22.00 |
Not yet formed |
Not yet formed |
|
M5 |
24.00 |
Not yet formed |
Not yet formed |
|
M6 |
contaminated |
contaminated |
contaminated |
M1 = Full strength of MS media without sugar; M2 = ½ (a half) strength of MS media without sugar; M3 = ¼ (fourth) strength of MS media without sugar; M4 = Full strength of MS media with 20 gL-1 of sugar; M5 = ½ (a half) strength of MS media with 20 gL-1 of sugar; M6 = ¼ (fourth) strength of MS media with 20 gL-1 of sugar; das = days after spores sowing. Each value was averaged from 5 replications.
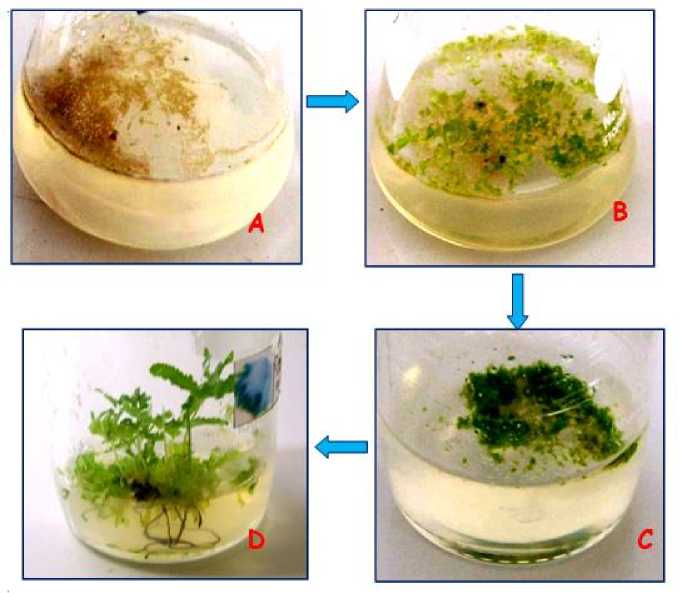
Figure 3. Growth of spores of L. circinnatum in-vitro
A= Spores after sowing; B= germinated spores; C=formation of prothalium; D=Sporophytes; Diameter of the bottom of the bottle = 4.5 cm
Table 2 shows results of the ex-vitro experiment. Up to 120 days after spores sowing (das), sporophytes were only formed on medium of paddy-silt soil and decomposed leaves either with or without addition of foliar fertilizer. We believed this is closely related to water content of the media. Analysis of water content was performed using method “water (%) by mass” (Icrisat, 2015) and can be seen in the Table 3. Paddy-silt soil had highest water content among types of medium used.
Lygodium is homosporous fern and has bisexual gametophyte. It has two free-living generation i.e. a haploid gametophyte and a diploid sporophyte (Lott et al. 2003). Sporophyte (young plant that formed from spores) of homosporous ferns can be produced through three types of mating, i.e. intragametophytic selfing (Soltis et al. 1988), intergametophytic crossing (Korpelainen and Kolkkala, 1996; Hooper and Hauffer, 1997) and mixing between both types (Soltis and Soltis, 1987). However, the mayority of them have intergametophytic crossing (Lott et al, 2003). In the current research we have no data about the type of mating of L. circinnatum, however, we believed that mating between an egg (usually in a structure of archegonium) and a sperm (in a structure named antheridium) in formation of sporophyte requires water, because sporophyte was not performed at the media of fine
grain soil and moss, in which the soil water content was low. Although media of decomposed leaves had water content less than 100%, however, it might contain more nutrients, so that sporophyte can performed in this type of media. Besides that, the height of sporophyte increased faster in decomposed leaf media when fertilizer was incorporated (from 1.20 cm to 2.03 cm) compared to paddy-silt soil media (from 1.91 cm to 2.08 cm) (Table 2), it might be nutrient at media of decomposed leaf to be available after 120 days and stimulated the growth of plant.
Figure 4. shows growth of L. circinnatum ex-vitro 120 days after spores sowing.. There were no sporophytes formed at media of fine grain soil (T1, T5) and moss (T4, T8) either with or without addition of fertilizer, indicated that these types of medium were not appropriate for growing L. circinnatum. Although spores germinated (formation of green-globular structures) and produced prothalium (heart-shape structures under microscopes) at media of fine grain soil and moss, however, sporophytes were failed to be formed. We suggested that mating between eggs and sperms did not occur at these types of medium. If we compared between in-vitro and ex-vitro experiments, we suggested, ex-vitro propagation was more appropriate, the technique was much easier and the spores grew faster compared to those of in-vitro.
Table 2. The Effects of medium on the growth of spores of L. circinnatum growing ex-vitro
|
Treatments |
Time of spore germination (das) |
Time of prothalia formation (das) |
Time of Sporophyte formation (das) |
Height of Sporophyte (cm) |
|
T1 |
20.50 bc |
29.75 b |
Not yet formed |
Not yet formed |
|
T2 |
19.75 bc |
28.13 b |
102.00 |
1.91 |
|
T3 |
21.00 bc |
29.88 b |
108.58 |
1.20 |
|
T4 |
26.00 a |
39.25 a |
Not yet formed |
Not yet formed |
|
T5 |
21,25 b |
29.25 b |
Not yet formed |
Not yet formed |
|
T6 |
19.00 c |
28.00 b |
111.75 |
2.08 |
|
T7 |
21.00 bc |
29.50 b |
115.75 |
2.23 |
|
T8 |
25.63 a |
36.13 a |
Not yet formed |
Not yet formed |
T1 = Fine grain soil, T2 = Paddy-silt soil, T3 = Decomposed leaves , T4 = Moss, T5 = Fine grain soil +1gkg-1 of foliar fertilizer, T6 = Paddy-silt soil +1gkg-1 of foliar fertilizer, T7 = Decomposed leaves +1gkg-1 of foliar fertilizer, and T8 = Moss+1gkg-1 of foliar fertilizer. The same letter behind values indicated not statistically different according to Duncan’t Multiple Range Test (DMRT) at level of 5% and vice versa. Each value was averaged from 10 replications.

Figure 4. Growth of spores of L. circinnatum at several types of medium ex-vitro.
Table 3. Water Content (%) of Media at Ex-Vitro Experiment (Dry Weight-Base)
|
Types of Media |
Water content (%) *) |
|
Fine grain soil Paddy-silt soil Decomposed leaves Moss |
59.85 210.81 71.13 62.27 |
*) The value was averaged from 3 samples
T1 = Fine grain soil, T2 = Paddy-silt soil, T3 = Decomposed leaves , T4 = Moss, T5 = Fine grain soil +1gkg-1 of foliar fertilizer, T6 = Paddy-silt soil +1gkg-1 of foliar fertilizer, T7 = Decomposed leaves +1gkg-1 of foliar fertilizer, and T8 = Moss+1gkg-1 of foliar fertilizer. Diameter of top of the pot = 15 cm.
Domestication of a wild fern of L. circinnatum was successfully done by using spores as planting materials. In conclusion, full strength of MS media without sugar was the most appropriate media for growth and development of spores of L. circinnatum in vitro. While for ex
vitro, the appropriate media were paddy silt-soil and decomposed leaf either with or without addition of foliar fertilizer. However, we suggested ex-vitro propagation was more appropriate, the technique was much easier and the spores grew faster compared to those of in-vitro.
Acknowledgement
The authors gratefully acknowledge the financial support provided by The Ministry of Research, Technology and Higher Education of Republic Indonesia through Competitive Grant Project (Hibah Bersaing).
References
Abou Dahab A M, Afaf MA, Habib YA., Hosni and Gabr AMM, 2005. Effect of MS-salt strength, sucrose and IBA concentration andacclimatization media on Ruscus hypoglossum L. micropropagation. Arab Journal of Biotechnology 8 (1): 141–154
Baligar VC. and Duncan RR. 1993. Crops as Enhancers of Nutrient Use. Academic Press Inc., San Diego, California. 574p.
Daud N, Taha RM., Noor NNM and Alimon H. 2011. Provision of low cost media options for in-vitro culture of Celosia sp. African Journal of Biotechnology 10 (80): 18349-18355
GBIF Backbone Taxonomy. 2015.
http://arctos.database.museum/name/Lygo dium %20circinnatum (download 27-092015)
Goolsby J.A, Wright AD and Pemberton RW. 2003. Exploratory surveys in Australia and Asia for natural enemies of old world climbing fern, Lygodium mycrophyllum : Lygodiaceae. Journal of Biological Control 28: 33-46.
Hooper E.A and Haufler AH. 1997. Genetic diversity and breeding system in a group of neotropical epiphytic ferns (Pleopeltis; Polypodiaceae). American Journal of Botany 84:1664-1674
Icrisat. 2015. Soil Moisture Calculation. (http://www.icrisat.org/what-we-do/learning- opportunities/lsu-
pdfs/Soil%20Moisture%20Calculation.pd f, download 27-09-2015)
Jeetendra N. and Manish B. 2011. Correlation of antioxidant activity with phenolic content and isolation of antioxidant compound
from Lygodium flexuosum (L.) SW extracts. Journal of Pharmacy &
Pharmaceutical Sciences 3 (2): 48-52
Korpalainen H and Kolkkala M. 1996. Genetic diversity and population structurein the outcrossing population of Equisetum arvense and E. hyemale (Equisetaceae). American Journal.of Botany 83: 58-62
Lott M.S, Volin JC, Pemberton RW and Austin DF. 2003. The production Biology of the invasive ferns Lygodium
microphyllum and L. japonicum (Shcizaeaceae): implication for invasive potential. American Journal of Botany 90(8): 1144-1152
Rahayu M. 2006. The accessment of Aplication of Manure Fertilizer on Growth of Lygodium circinnatum.
(http://www.Lygodium Research/Paku
ketak,download 01/02/2009) (In
Indonesian)
Raka INN, Artha IN and Semarajaya CGA. 1998. Conservation of Lygodium germplasm. Research Report, collaboration between Deptartment of Agronomy, Faculty of Agriculture, Udayana University and KEHATI (Non Government
Organisation), Denpasar. (In Indonesian)
Saad AIM and Elshahed AM. 2012. Plant tissue culture media. http://creative
commons.org./licenses/by/3.0 (download 03/01/2015)
Soltis D and Soltis P 1987. Breeding system of the fern Dryopteris expansa: evidence for mixed mating. American Journal.of Botany 74: 504-509
Soltis, P, Soltis D and Holsinger K. 1988. Estimates of intragametophytic selfing and interpopulational gene flow in homosporous ferns. American Journal.of Botany 75: 1765-1770
Yamauchi T, Oyama N, Yamane H, Murofushi N, Schraudolf H, Pour M, Furber M and Mander LN. 1996. Identification of antheridiogens in Lygodium circinnatum and Lygodium flexuosum. Plant
Physiology. 111: 741-745
138
Discussion and feedback