SUPPRESSION ABILITY OF CRUDE EXTRACT DERIVED FROM MARINE BIOTA AGAINST FUSARIUM OXYSPORUM F.SP. VANILLAE
on
JURNAL BIOLOGI XIV (I) : 7 - 10
ISSN : 1410 5292
SUPPRESSION ABILITY OF CRUDE EXTRACT DERIVED FROM
MARINE BIOTA AGAINST Fusarium oxysporum f.sp. vanillae
I KETUT SUADA, NI WAYAN SUNITI Study Program of Agroecotechnology, Faculty of Agriculture, Udayana University, Bali
ABSTRACT
The objective of this research was to investigate suppression ability of marine biota extracts against Fusarium oxysporum f.sp. vanillae of vanilla stem rot. Samples were collected at intertidal zones and in the depth of 1-7 m from seven beaches in Bali. Screening of active compounds of biota extracts were conducted using inhibition zone of well diffusion method on Potato Dextrose Agar (PDA). The extract was tested in-vitro in PDA medium using completely randomized design with three replicates. The methanolic extract of Aglaophenia sp. was able to suppress the growth of F. oxysporum f.sp. vanillae effectively, with minimum inhibition concentration (MIC) of 0.05 %. The extract inhibited colony growth diameter and total mycelial dry weight.
Keywords: Fusarium oxysporum f.sp. vanillae, Aglaophenia sp., well diffusion method.
INTISARI
Penelitian ini bertujuan untuk mengungkapkan aktivitas antimikroba ekstrak biota laut terhadap Fusarium oxysporum f.sp. vanillae penyebab busuk batang pada tanaman vanili. Sampel biota diambil dari daerah intertidal dengan kedalaman 1-7 m pada 7 pantai di Bali. Skrining aktivitas antibiotik dilakukan menggunakan zone hambat dengan metode difusi sumur pada media PDA. Pengujian dilakukan secara in vitro dengan rancangan acak lengkap dengan tiga ulangan. Penelitian ini membuktikan bahwa ekstrak methanol Aglaophenia sp. mampu menekan pertumbuhan F. oxysporum f.sp. vanillae dengan konsentrasi hambat minimum (MIC) 0,05%. Ekstrak ini menekan diameter pertumbuhan koloni dan total berat kering miselium.
Kata kunci: Fusarium oxysporum f.sp. vanillae, Aglaophenia sp., metode difusi sumur
INTRODUCTION
The increase of international competitive on trading triggers the effort of producers to produce vanilla beans in high quality without the presence of recidual pesticides. Controlling Fusarium oxysporum f.sp. vanillae, (the pathogen of vanilla stem rot) using sinthetic compounds would result in high pesticide residue on agricultural products. This method is no longer accepted in a sustainable agriculture (Hadisutrisno, 2004).
The use of natural product derived from marine organism is promising, because the bioactive compound is generally safe and biodegradable. More over, the secondary metabolites from marine organisms are unique and often better than those of terrestrial biota (Anggadiredja, 2004). Those facts trigger people to explore antimicrobes from marine biotas particullarly from sedentary organisms. These organisms could not hinder from predator, therefore they develop defence mechanism by producing secondary metabolites including antifungial materials.
Some antibiotics derived from marine macro- and micro-algae have been developed, such as trichlorethane
and perchlorethane (Marasneh et al., 1995). Some antifungi also have been extracted from marine biotas such as Sargassum despiense, Turbinaria decurrense, Cystoseira tamariscifolia and able to suppress Fusarium, Penicillium, and Aspergillus sp. (Mabrouk et al., 1985; Souhaili et al., 2004).
At the intertidal zones of Serangan and Canggu beach, Bali, 25 species of algae, 27 sponges, and 7 coralls have been collected. The extract of these biotas has been fpund to have an inhibition activity against some fungi and bacteria (Suada et al., 2008).
The purpose of this research was to investigate the suppression activities of the extract derived from marine biotas against Fusarium oxysporum f.sp. vanillae (Fusarium), the causal agent of vanilla stem rot.
MATERIALS AND METHODS
Strain of Fungi and Marine Biota Samples
Fusarium (Fusarium oxysporum f.sp. vanillae) was isolated and identified in Agricultural Biotechnology Laboratory, Udayana University from infected vanilla plants collected from the field in Tabanan, Bali. Single
Naskah ini diterima tanggal 27 Maret 2010 disetujui tanggal 7 Mei 2010
spore isolation technique was applied to obtained single strain of Fusarium. Samples of Marine biotas were collected at intertidal zones in the depth of 1-7 m at seven beaches (Serangan, Batu Bolong, Tanah Lot, Menjangan, Lovina, Tukad Abu, and Nusa Penida) of Bali Province.
Medium and Culture Conditions
The medium used for isolation of Fusarium was Matuo medium (Matuo, 1972). The medium consisted of 1.00 g K2PO4, 0.50 g KCl, 0.50 g MgSO4.7H2O, 0.01 g Fe-Na-EDTA, 2.00 g L-Asparagine, 20.00 g D-Galaktose, and 1.00 L aquadest. The medium was sterilized at 121oC for 20 min. Antimicrobe substances were added by 1.00 g PCNB 75% WP, 0.50 g Oxgall, 1.00 g Na2B2O7, and 0.30 g Streptomycin sulfate. Fusarium were then cultured in potato dextrose agar medium (PDA, Nissui) and potato dextrose broth (PDB, DIFCO) pH 4.5.
Well Diffusion Method
Two mililitres of spore suspension of Fusarium (2x105 conidia/ml) were placed into petri dishes, then 20 ml PDA were added , shaked gently until the conidia well mixed. Over the solid medium of PDA wells were made by cork borer Ø 5 mm. The wells were filled with 40 µl conidium extracts in various concentrations , then incubated at room temperature for 48 h (Berghe and Vlietinck, 1991; Rios et al., 1988). The inhibition zone appeared on the medium was examined. Through this experiment the minimum inhibition concentration (MIC) was determined.
Fresh Biomass Extraction
Two grams of marine biotas (Figure 1, Table 1) collected from 7 beaches were chopped, ground, then 5 ml solvents were added for maceration. Solvents used in this experiment were n-hexan, chloroform, aceton, ethanol, methanol, and water. The filtrate was put in eppendorf tubes, centrifuged at 5000 rpm and the supernatan was transferred into new eppendorf tubes. The filtrate aerated and bioassayed using well diffusion method (as described above) against Fusarium with the concentration of 50% (w/v) on PDA medium.
Dry Biomass Extraction
The extract Aglaophenia sp. was found as the most active extract and showed the widest inhibition zones toward the growth of Fusarium sp. Its dry material was then tested. The biota biomass was air dried (up to 12% water content, undirect sun light), then ground into powdered. Maceration of the powdered biotas was diluted in methanol at the ratio of 1:2 (w/v) at room temperature for 24 h. The filtrate was evaporated in vacuum rotary evaporator at 40oC. The crude extract obtained was freeze dried.
Inhibition on Colony Growth Diameter
Fusarium colony plug, 5 mm in diameter, of 3 days culture was placed on the centre of PDA medium in Petri dish in various extract concentrations (0, 0.05, 0.10, 0.15, and 0.20%). The cultures were incubated for 7 days at room temperature (fungi on controlled culture were growing and covered all surface of the medium). The diameter of growing colonies was measured daily.
Inhibition of Mycelial Dry Weight
The mycelia of the treatments above were harvested by adding 1% HCl, then oven dried at 80oC, up to constant weight. Mycelia harvested from every treatments above were weighed.
This experiment was also run in PDB liquid medium. The cultures were incubated for 7 days at room temperature and the mycelia were filterred using Whatmann paper no 2. The mycelia were oven dried at 80oC up to constant weight . All data were analysed with Complete Random Design and the treatments were analised using DMRT 5% differentiation.
RESULTS
Diversity of Marine Biotas Collected from Bali Beaches
Fifty three types of marine biotas belong to algae, sponges, and hydrozoa were found and collected from seven beaches in Bali. Twelve biotas exhibit antifungi against Fusarium e.g.: algae: Gracilaria arcuata, Eucheuma cottonii; spongess: Haliclona sp., Tedaria ignis, and Spongilla sp.; corals: Aglaophenia sp. (Figure 1; Table 1).
MIC is the smallest concentration an extract that would inhibit the growth of microbes visually after two days incubation (Andrews, 2006). MIC is refers to susceptibility of microbe against ones extract. According to Hoffmann et al. (1993) and Andrews (2006), the MIC extract less than 0.1% is reliable to be tested. Forexample, if the extract of Aglaophenia sp. in methanol exhibited inhibition zone of 58 mm, then its MIC less than 0.1% was tested for the next steps.
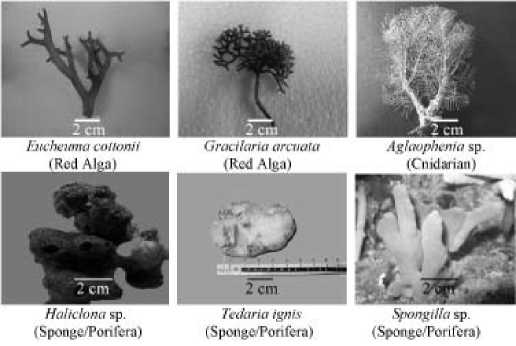
Figure 1. Some marine organisms that exhibited antifungal activity against Fusarium.
Suppression Ability Of Crude Extract Derived From Marine Biota Against Fusarium oxysporum f.Sp. Vanillae [I Ketut Suada, Ni Wayan Suniti]
Table 1. Diameter of inhibition zones and MIC values of marine biota
extract against Fusarium
|
Number |
Species |
Diameter of inhibition zone (mm) |
MIC value (%) |
Beaches of collections |
|
Ethanol extracts of marine algae | ||||
|
1 |
Eucheuma cottonii |
9 (fare) |
0.4 |
Serangan |
|
2 |
Gracilaria arcuata |
18 (strong) |
0.2 |
Serangan |
|
3 |
Gracilaria arcuata |
19 (strong) |
0.2 |
Batu Bolong |
|
4 |
Gracilaria arcuata |
17 (strong) |
0.2 |
Tanah Lot |
|
Methanol extract of sponges (Porifera) | ||||
|
1 |
Amorphinopsis sp. |
15 (strong) |
0,5 |
Tukad Abu |
|
2 |
Family:Tetillidae |
10 (fare) |
5.0 |
Menjangan |
|
3 |
Haliclona sp. |
18 (strong) |
0.2 |
Tukad Abu |
|
4 |
Family:Raspailiidae |
19 (strong) |
0.4 |
Menjangan |
|
5 |
Tedaria ignis |
18 (strong) |
0.2 |
Tukad Abu |
|
6 |
Spongilla sp. |
23 (very strong) |
0.2 |
Tukad Abu |
|
7 |
Clathrina sp. |
9 (fare) |
5.0 |
Tukad Abu |
|
Methanol extract of coral (Cnidaria) | ||||
|
1 |
Aglaophenia sp. |
58 (very strong) |
0.05 |
Tukad Abu |
Legends :
• Inhibition zones was based on concentration of 50%
• Inhibition zones (diameter of inhibition zone sustracted by diameter of extract well) in PDA were incubated for 24 hours at room temperature.
• Category in bracket ( ) was inhibition ability based on Davis Stout Categories (Ardiansyah, 2005).
• Results were based on 5 replicates
• Standard MIC used for antimicrobe was ≤ 0.1% (w/v) (Hoffmann et al., 1993; Andrews, 2006).
Crude extract of Aglaophenia sp. was able to inhibit the patogen growth in-vitro on PDA medium. Well diffusion method exhibited its MIC against Fusarium spores was 0.05% after two days incubation.
According to LIPI (The Indonesian Institute of Science ) identification, the Aglaophenia sp. contains the most active compound as the marine animal, and its classification is as follows: Kingdom Animalia, Phylum Cnidaria (Coelenterata), Class Hydrozoa, Ordo Hydroida, Subordo Thecata, Family Aglaopheniidae, Genus Aglaophenia, Spesies Aglaophenia sp.
The Effect of Extract Against Colony Growth
The inhibition of the extract against the colony growth differed significantly among concentrations of the treatments. The percentage of inhibition increased in increase of the extract concentrations.
The colony was significantly inhibited on 0.05% extract concentration, and the entire inhibition occurred on 0.15% (w/v) as the value of MIC inhibition criteria based on mycelium (Figure 2; Table 2).
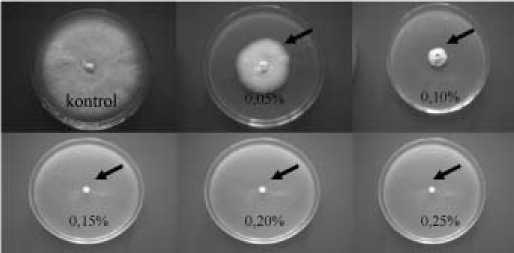
Figure 2. Effect of crude extracts against mycelium of Fusarium in PDA medium. %= tested concentration. Sign (^) is the affected colony after 7 days incubation.
Table 2. Development of Fusarium colony diameters affected by various concentrations of Aglaophenia sp.
|
Concentration (%) |
Diameter of colony (mm) in days after inoculation | ||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 | |
|
0.00 |
6.00 a |
13.33 a |
38.67 a |
54.67 a |
68.33 a |
75.67 a |
82.67 a |
|
0.05 |
1.00 b |
6.33 b |
18.00 b |
24.67 b |
30.33 b |
35.33 b |
39.33 b |
|
0.10 |
0.00 c |
1.33 c |
8.00 c |
10.33 c |
12.00 c |
13.67 c |
16.00 c |
|
0.15 |
0.00 c |
0.00 d |
0.00 d |
0.00 d |
0.00 d |
0.00 d |
0.00 d |
|
0.20 |
0.00 c |
0.00 d |
0.00 d |
0.00 d |
0.00 d |
0.00 d |
0.00 d |
Legends:
Numbers followed by the same letters in one column were not significantly different according to Duncan Multiple Ring Test of 5%. Data analyzed were √(x+0,5) transformed.
The inhibition patterns of the extract against Fusarium colony growth were presented in Figure 2. The inhibition ability was stable above 50% from the second days to seven days of observations. The inhibition patterns indicated that the bioactive compound was stable for long periods of time which means that the extract protection for the plants was potentially good.
Effect of The Extract Against Mycelia Dry Weight
Effect of extract against mycelia dry weight is listed on Table 3. The extract was significantly influenced the dry weight of mycelia.
The higher extract concentration applied to the fungi, the higher inhibition happened. For example, the inhibition ability of mycelia dry weight on concentration of 0,15% was significantly higher than the lower concentration (e.g. 0.10%).
Table 3. Inhibition ability of Aglaophenia sp. extract of mycelia dry weight of the Fusarium
|
Concentration (%) |
Tested in PDA |
Tested in PDB | ||
|
Dry weight (mg) |
Inhibition ability (%) |
Dry weight (mg) |
Inhibition ability (%) | |
|
0.00 |
59.91 a |
0.00 a |
23.93 a |
0.00 a |
|
0.05 |
8.72 b |
85.50 b |
15.63 b |
34.33 b |
|
0.10 |
6.86 c |
88.61 c |
7.20 c |
69.85 c |
|
0.15 |
1.34 d |
97.83 d |
0.63 d |
97.33 d |
|
0.20 |
0.82 d |
98.72 d |
0.83 d |
96.55 d |
Legends:
Numbers followed by the same letters in one column were not significantly different according to DMRT 5%. Data analyzed were log (x+1) transformed.
The Form of Extract Inhibitions on Fusarium
One symptom of extract inhibition detected on mycelium was wrinkled. The symptom was caused by plasmolysis processes on mycelium, especially on young mycelium (Figure 3).
DISCUSSION
Biodiversity of marine biota found at Bali beaches containing bioactivity compounds were 12 species that comprised of algae, sponges and corrals. The most effective one was animal marine biota Aglaophenia sp. with MIC value of 0.05% based on inhibition on germination spore of Fusarium. Based on Hoffmann et al. (1993) and Andrews (2006) criterias, the extract with MIC less than 0.1% is reliable to be tested, therefore
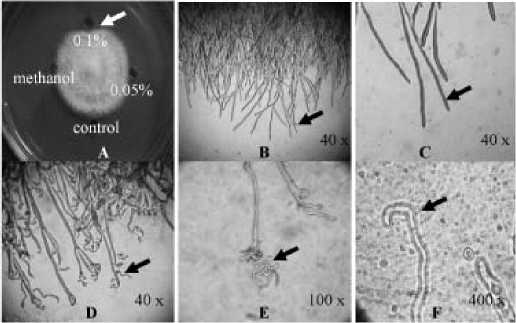
Figure 3. The effect of Aglaophenia sp. extract on Fusarium mycelia. A: Colony affected by extract at concentrations of 0%, 0.05%, 0.1% (w/v) and methanol; B and C: Control; D, E, and F: abnormal mycelia: spirally wrinkled at 0.1% concentration.
methanol extract of Aglaophenia sp. could be tested for the next steps.
Figure 2 and Table 2 showed that the extract significantly inhibited the colony growth and total inhibition (100%) occurred at concentration of 0.15%. The inhibition occurred up to day seventh of treatments. This result suggested that the extract is fungitoxic.
The extract significantly suppressed the dry weight of mycelia compared to control. The higher the concentration of treatments, the higher the suppression against mycelial dry weight. The mycelial dry weight at 0.15% was significantly lower than other treatments. The mycelia on treatments 0.15% and above were not able to grow. Therefore, the mycelia dry weight counted at treatments 0.15% and 0.20% was the mycelia from the original inoculums. This means that the extract was able to inhibit Fusarium at the mycelium stages.
One symptom of inhibition on mycelium growth was the wrinkled of mycelium which was caused by plasmolysis that occurred particularly on young myceliua (Figure 3). According to Sugiharso (1980) that bioactive compounds of fungicides commonly cause plasmolysis on Fusarium. The bioactive compound acts as chelating agent of membrane molecule and caused leaking in membrane cell. Consequently, K+, Na+, Cl-, Ca+2 ions from cytoplasm come out of the cell. This could happen due to the outer cell osmotic pressure was higher than inner cell. Therefore water run out of cell and cell then becomes dry and wrinkled. Furthermore, Stenersen (2004) stated that the common symptoms caused by antifungi compound are distortion of cell, swelling, and spiraly wrinkling cell.
CONCLUSSION
Among all marine biotas collected from Bali island beaches there were 12 species showed inhibition activity against Fusarium oxysporum f.sp. vanillae. The most active extract was from Aglaophenia sp. with MIC of 0.05%. The extract was able to suppress colony growth and mycelial dry weight. The mycelia affected by the extract were wrinkled.
ACKNOWLEDGEMENT
We would like to thank Mr. Sekar and friends for their kind assistant on sample collection.
REFERENCES
Andrews, J. M. 2006. Determination of Minimum Inhibitory Concentration (MIC). Department of Microbiology Birmingham. 19p.
Anggadiredja, J. T. 2004. Diversity of Antibacterial Substances from Selected Indonesian Seaweeds . Dissertation. UI-Jakarta.
Ardiansyah. 2005. Daun Beluntas Sebagai Bahan Antibakteri dan Antioksidan. http://www.beritaiptek.com/cetak-berita. php?kat= berita&id=60 [16/12/2006].
Berghe, D.A.V. and A.J. Vlietinck. 1991. Screening Methods for Antibacterial and Antiviral Agent from Higher Plant In Dey, P.M. and J.B. Harborne (Eds.): Methods in Plant Biochemistry Vol. 7. Academic Press, London.
Hadisutrisno, B. 2004. Budidaya Vanili Tahan Busuk Batang. Swadaya, Jakarta. 87p.
Hoffmann, J.J., B.N. Timmermann, B.N. Meclaughlin, H. Punnapayak. 1993. Potential Antimicrobial Activity of Plant from Southwestern United States. Int. J. Pharmacol. 31(2):101-115.
Mabrouk, S. S., N. M. A. El-Shayeb, A.H. El-Refai, L. A. R. Sallam, A. A. Hamdy. 1985. Inhibitory Activities of Some Marine Algae on Aflatoxin. Appl. Microbiol. Biotechnol. 22(2):152-155.
Marasneh, I., M. Jamal, M. Kashasneh, M. Zibdeh. 1995. Antibiotic Activity of Marine Algae Against Multi-Antibiotic Resistant Bacteria. Microbiology 83:23-26.
Matuo, T. 1972. Taxonomic Studies of Phytopathogenic Fusaria in Japan. Rev. Plant Protec. Res. 5: 34-45.
Rios, J.L., M.C. Recio, A.Villar. 1988. Screening Methods for Natural Product with Antimicrobial Activity (A Review of Literature). J. Ethnopharmacol. 23:127-149.
Souhaili, Z, M. Lageouli, M. Faid, Fella. 2004. Inhibition of Growth and Mycotoxin Formation Moulds by Marine Alga Cystoceira tamariscifolia. Afr. J. Biotechnol. 3(1):71-75.
Stenersen, J. 2004. Chemical Pesticides: Mode of Action and Toxicology. CRC Press, Boca Raton, London, New York, Washington D.C. 276p.
Suada, I.K., I.G.P. Wirawan, W. Adiartayasa. 2008. Uji Antimik-roba Ekstrak Beberapa Jenis Alga Laut dari Pantai Serangan dan Canggu, Bali. Agritrop 27(2):55-62.
Sugiharso. 1980. Fungisida. Departemen Ilmu Hama dan Penyakit Tumbuhan, Fakultas Pertanian, IPB, Bogor. 94p.
10
Discussion and feedback