Antibiotics Resistance Level of Vibrio spp. Isolated From Northern Bali Area
on
Advances in Tropical Biodiversity and Environmental Sciences 4(2): 30-34, September 2020 e-ISSN: 2622-0628
DOI: 10.24843/ATBES.2020.v04.i02.p01 Available online at: https://ojs.unud.ac.id/index.php/ATBES/article/view/59182
30
Antibiotics Resistance Level of Vibrio spp. Isolated From Northern Bali Area
Putu Widya Purnama Dewi1*, Pande Gde Sasmita Julyantoro1, I Wayan Darya Kartika1
1Department of Aquatic Resources Management, Faculty of Marine and Fisheries, Udayana University Jl. Kampus Unud Bukit Jimbaran, Kuta Selatan, Badung, Bali
*Corresponding author: widyapd8@gmail.com
Abstract. One conventional method that usually done when the organism infected by pathogenic bacteria is using antibiotics, either with single or combination usage. However, the misuse of antibiotics dosages leads to resistance development of pathogenic bacteria. This study aims to determine the antibiotic resistance level of Vibrio spp. which was isolated from the waters of North Bali and to investigate the difference of resistance level between Vibrio spp. isolated from the cultivation area and outside the cultivation area. This research was conducted at the Microbiology Laboratory of BKIPM Denpasar and the Laboratory of Fisheries at the Faculty of Marine Science and Fisheries, Udayana University from November 2019 to January 2020. Antibiotic tests were carried out in vitro using 8 types of antibiotics namely tetracycline, oxytetracycline, enrofloxacin, ciprofloxacin, amoxycillin, doxycycline, ampicillin, and erythromycin with different concentrations of 0.5, 1, 2, 4, 8, 16, 32, 64, 64, 128, 256 and 512 ppm. and this test was carried out using a microplate reader to obtain absorbance values before and after incubation to determine the level of resistance of isolated Vibrios. The research showed that overall minimum inhibitory concentration (MIC) of Vibrio was below 100 ppm while the Vibrio spp. isolated from the cultivation area has higher resistance levels compared to outside cultivation areas.
Keywords: in vitro; minimum inhibitory concentration (MIC); water quality parameter
-
I. INTRODUCTION
Aquaculture is an activity to produce aquatic organisms in a controlled environment in order to get profit. There are several problems occur in aquaculture, one of them is disease outbreaks that have a significant influence on this sector. One genus of bacteria that can cause disease in fish is Vibrio spp. [1]. Vibrio spp. is a type of gram-negative bacteria that are facultative anaerobes which means they can survive either with or without oxygen. Vibrio spp. classified into several types which are the main pathogens in fish and shrimp, namely Vibrio alginolyticus, V. damsela, V. charcariae, V. anguilarum, V. ordalli, V. cholera, V. salmonicida, V. vulnificus, V. parahaemolyticus, V. harveyi, V. pelagia, V. splendidus, and V. fishceri [2]. The use of antibiotic are conventionally implemented to cure bacterial diseases caused by pathogenic Vibrio in the cultivation area. These antibiotics are usually used for disease prevention and treatment [3]. An antibiotic is a substance produced by a microorganism that can inhibit the growth of other microorganisms in very small amounts. Now, antibiotics are still often used as the first option for the treatment of bacterial infectious diseases. The action mechanism of antibiotics usually done in four ways, including inhibition of cell wall synthesis, inhibition of nucleic acid synthesis, inhibition of cell membrane function and inhibition of protein synthesis [4]. However, the use of these
antibiotics continuously with inappropriate dosages can cause negative effects for the environment, fish and humans. In addition, it can cause resistance and also residues in fish and antibiotic content in fish commodities affected the decrease in fish prices in the international market [5]. Bali is one of the centers of tourism in Indonesia. Therefore, the waters are important to be maintained so that it can’t be contaminated by antibiotic residues. This study was conducted to determine the current condition of the level of antibiotic resistance of Vibrio spp. around aquaculture areas in North Bali by comparing between inside and outside of the aquaculture area.
-
II. METHODS
Experimental setup
This research is experimental with three repetitions for each treatment. Antibiotic tests were carried out in vitro using 8 types of antibiotics namely tetracycline, oxytetracycline, enrofloxacin, ciprofloxacin, amoxycillin, doxycycline, ampicillin, and erythromycin with different concentrations of 0.5, 1, 2, 4, 8, 16, 32, 64, 64, 128, 256 and 512 ppm.
Materials
The material used were water samples taken from three locations in the waters of North Bali (Sanggalangit
Village, Sukadana Village and Patas Village). The culture media used were Thiosulfat Citrate Bile Salts Sucrose Agar (TCBSA) and Nutrient Agar (NA). Antibiotics were tested using eight types including tetracycline, oxytetracycline, enrofloxacin, ciprofloxacin, amoxycillin, doxycycline, ampicillin, and erythromycin. The tools used in this research are laminary airflow, incubator (Memmert UNB 400), microplate, microplate reader (Zenix 320), and autoclave (Hiclave HL Series).
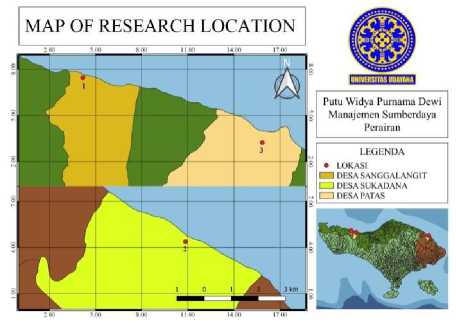
Figure 1. Sampling Location
Preparation of tested bacteria
Bacteria were cultured on Thiosulfate Citrate Bile Salts Sucrose Agar (TCBSA) media at 37°C for 24 hours and then purified with yellow and green colonies on Nutrient Agar (NA) media for 24 hours. After 24 hours, a presumptive test and biochemical test were carried out to determine the type of Vibrio bacteria that was obtained. Then a bacterial suspension was made with a turbidity level adjusted to McFarland 0.5 (108 CFU / mL) visually and then diluted until dilution 107 [6] then antibiotic testing was carried out for this bacterial suspension.
Antibiotic Testing
Antibiotics were added to plate 96 well as much as 140 µL then 10 μL of bacterial suspension was added to each well, and then put it into a microplate reader with a wavelength of 450 nm before incubation [7] to determine the initial absorbance value. After being incubated for 24 hours, put back into the microplate reader with the same wavelength to find out the absorbance value after incubation.
Determination of Minimum Inhibitory Concentration
Minimum Inhibitory Concentration is the lowest concentration of antibiotics that inhibits the growth of microorganisms and is carried out in vitro. This research was conducted by looking at the difference in value from the absorbance values after and before incubation. If the
results show the (-) value at the lowest concentration, it indicates that the bacteria is sensitive to the antibiotic tested and that is the MIC value of the antibiotic.
Water Quality Parameter
The parameters measured in the study are temperature, DO, pH, and salinity. Measurement of temperature, pH and salinity were measured at the time of taking water samples. DO measurements were carried out ex-situ using the Winkler method at the Fisheries Laboratory of Udayana University.
Data Analysis
Data analysis used in this research is descriptive in tables and graphs. obtained data are absorbance values that disputed from the after and before incubation values. Then the percentage is processed descriptively [8] as follows:
RL (%) = I x 100 %
Noted: RL is Resistance level (%), a is the amount of antibiotic concentration with resistant bacteria and b is the amount of concentration tested.
-
III. RESULT AND DISCUSSION
Identification of Vibrio spp.
Based on the results of presumptive and biochemical tests, 6 types of bacteria were identified, namely V. alginolyticus, V. parahaemolyticus, V. cincinnatiensis, V. vulnificus, V. hollisae, and V. harveyi. The identification of these bacteria refers to the Determinative Bacteriology [9] and Biochemical Test for Identification of Medical Bacteria [10]. In biochemical testing, each type of Vibrio found has different traits. Whereas the presumptive test has the same characteristics in each type [11].
TABLE I
VIBRIO BACTERIA FOUND IN NORTHERN BALI WATERS
|
Bacterial code |
Bacterial name |
|
A1 K |
V. alginolyticus |
|
A1 H |
V. parahaemolyticus |
|
A2 K |
V. cincinnatiensis |
|
A2 H |
V. vulnificus |
|
B1 K |
V. cincinnatiensis |
|
B1 H |
V. parahaemolyticus |
|
B2 K |
V. hollisae |
|
B2 H |
V. harveyi |
|
C1 K |
V. cincinnatiensis |
|
C1 H |
V. parahaemolyticus |
|
C2 K |
V. cincinnatiensis |
|
C2 H |
V. parahaemolyticus |
Noted: K is a yellow colony, H is a green colony, A1 is bacteria in the first location ponds, A2 is bacteria outside first location ponds, B1 is bacteria in the second location ponds, B2 is bacteria outside second location ponds, C1 is
bacteria in the third location ponds and C2 is bacteria outside third location ponds.
Antibiotic Testing
The results of the antibiotic test with the type of antibiotic with several concentrations tested showed that the V. parahaemolyticus found in 3 locations of North Bali waters in the cultivation area and outside the cultivation area had the highest resistance level for each antibiotic tested (Figure 2.). At the first location, this bacterium was found in the cultivation area and had a percentage value ranging from 72.7-100% with an average percentage of 84.08%. In the second location, this bacterium was found in the cultivation area and had a percentage value ranging from 54.5 to 90.9% with an average percentage of 69.3%. The overuse of antibiotics in aquaculture not only increases the selection of antibiotic-resistant bacteria and the dissemination of the antibiotic-resistant genes but also results in the presence of antibiotic residues in aquatic organisms such as fish. The statement was also reported by Lopatek et al [12], moreover, in water environments, different microorganisms, including V. parahaemolyticus, are able to exchange their genetic determinants, which may also be the cause of increasing resistance to antibiotics.
This species is also found in the third location in the cultivation area and outside of the cultivation area with a resistance level of 100%. Other types of bacteria that have a high level of resistance are V. hollisae, which have a percentage value ranging from 54.5-100%. This is also similar to the research of Hickman et al [13], that in antibiotic tests, V. hollisae is resistant and this type of Vibrio can be obtained in human feces who ate raw processed seafood. Antibiotics resistant bacteria can occur because of the inappropriate antibiotics dosing and irregular period of dosing. This statement was also reported by Apriliani et al [14]. that the use of inappropriate antibiotic doses can cause resistant bacteria.
According to Caroll et al [15], bacteria have a mechanism that makes these bacteria resistant to antibiotics, that they can produce enzymes that destroy the active substances inside the drug and change its permeability to that drug. Another opinion, according to Sanchez and Demain [16], is that bacteria found in cultivation areas are often exposed to several tested antibiotics so that they produce resistant genes derived from antibiotic-producing strains. the gene can protect itself from the exposure and then the resistant gene has the potential to be transferred to other bacteria.
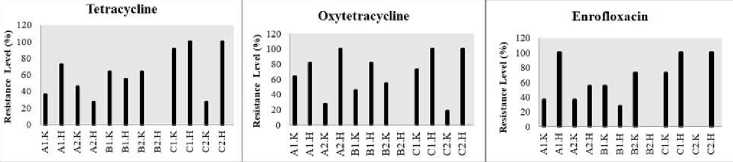
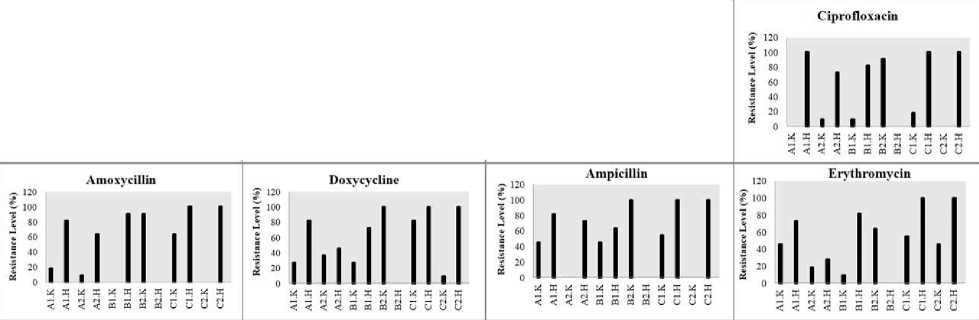
Figure 2. Resistance Level of Vibrio spp. on Each Antibiotics
Determination of Minimum Inhibitory Concentration
Based on the overall results of antibiotic testing, it was found that the MIC of all antibiotics tested was below the average concentration of 100 ppm. This can be seen from the absorbance value which tends to decrease so produce the value (-). Based on this, it can be stated that all the antibiotics tested were quite strong in inhibiting bacterial growth. In line with the opinion of Silva et al [17], the antibiotic activity can be divided into 4 groups based on MIC, namely MIC <100 ppm (strong),
MIC 100-500 ppm (moderate), MIC 500-1000 ppm (low) and MIC > 1000 ppm (inactive).
Water Quality Parameter
The measured water quality parameters are at the optimal level for seawater cultivation. It is suspected that the bacteria in the three locations do not have a correlate with the condition of water quality in the cultivation area. Vibrio bacteria arise because they already exist in these aquaculture areas [18].
TABLE II
WATER QUALITY MEASUREMENT RESULT
|
Sampling Location |
Water Quality | |||
|
Temperature (°C) |
DO (mg/L) |
pH |
Salinity | |
|
1 Cultivation area |
32 |
5.5 |
7.9 |
27.7 |
|
Outside area of cultivation |
32 |
5.9 |
8 |
28.3 |
|
2 Cultivation area |
29.7 |
7.9 |
8.1 |
20.7 |
|
Outside area of cultivation |
30 |
8.3 |
7.9 |
26 |
|
3 Cultivation area |
31.2 |
7.6 |
8.3 |
33.3 |
|
Outside area of cultivation |
35.7 |
8.4 |
8.2 |
25.3 |
-
IV. CONCLUSION
This study concluded that the overall minimum inhibitory concentration (MIC) of Vibrio isolated from the North Bali area was below 100 ppm, while Vibrio spp. isolated from farm areas have a higher level of resistance compared to the bacteria that found from outside farm areas and bacteria which are isolated from cultivation areas have higher resistance compared to bacteria in areas outside of cultivation.
ACKNOWLEDGEMENTS
We acknowledge Balai Karantina Ikan, Pengendalian Mutu dan Keamanan Hasil Perikanan (Balai KIPM) Denpasar, Bali and research team for all kind contribution to this research.
REFERENCES
-
[1] Chatterjee S, S Haldar. 2012. Vibrio Related Diseases in Aquaculture and Development of Rapid and Accurate Identification Methods. J. Marine Sci Res Dev. 1-7.
-
[2] Fahri M. 2008. Pathogenic Bacteria in Aquaculture of Vibrio parahaemolyticus. Postgraduate Program in Aquaculture, Brawijaya University, Malang. pp 66.
-
[3] De Melo R, LM Almeida, DE Hofer, CM Falavina dos Reis, GND Theophilo, AFDM Santos, RHSDF Vieira. 2011. Antibiotic resistance of Vibrio parahaemolyticus isolated from pondreared Litopenaeus vannamei marketed in Natal, Brazil. Brazilian Journal of Microbiology, 42: 1463-1469.
-
[4] Waluyo L. 2010. Basic Microbiological Methods Techniques. UMM Press. Malang.
-
[5] Kusmarwati A, Y Yennie, N Indriati. 2017. Antibiotic Resistance in Vibrio Parahaemolyticus from Vaname Shrimp from the North Coast of Java for the Export Market. Center for Research on
Product Processing and Biotechnology, Marine and Fisheries. Jakarta Pusat.
-
[6] Sutton S. 2011. Measurement of Microbial Cells by Optical Density. Journal of Validation Technologi. 17: 46-49.
-
[7] Astuti SD, IR Djoni, Ni’matuzahroh, M Zainuddin, Suhariningsih. 2011. Photodynamic Potential of Inactivation of Staphylococcus aureus and Vibrio cholerae with Endogenous Photosensitizer on Irradiation of Blue (430 ± 4) nm and Red (629 ± 6) nm. Berk. Penel. Hayati, 16: 127-131.
-
[8] Ridwan. 2004. Survey Research Methods. Pustaka LP3ES. Jakarta.
-
[9] Holt JG, NR Kreig, PHA Sneath, JT Staley, ST Williams. 1994. Bergey’s Manual of Determinative Bacteriology. Ninth Ed. A Wolters Kluwer Company. Philadelphia. Pp. 562-570.
-
[10] Faddin JFM. 1980. Biochemical Test for Identification of Medical Bacteria, London, William and Wilkins, pp. 36-40.
-
[11] Sarjito M, C Apriliani, AH Haditomo. 2015. Agent that causes Vibriosis in Vaname Shrimp (Litopenaeus gariepinus) Intensively Cultivated in Kendal. Jurnal Kelautan Tropis, 18 (3): 189-196.
-
[12] Lopatek M, K Wieczorek, J Osek. 2018. Antimicrobial Resistance, Virulence Factors and Genetic Profiles of Vibrio parahaemolyticus from Seafood. Applied and Environmental Microbiology, Vol. 84 (16).
-
[13] Hickman FW, JJ Farmer, DG Hollis, GR Fanning, AG Steigerwalt, RE Weaver, DJ Brenner. 1982. Identification of Vibrio hollisae sp. nov. from Patients with Diarrhea. Journal of Clinical Microbiology, 15(3): 395-401.
-
[14] Apriliani M, Sarjito, AH Haditomo. 2016. Diversity of agents that cause Vibriosis in Vaname Shrimp (Litopenaeus vannamei) and their sensitivity to antibiotics. Journal of Aquaculture Management and Technology, 5(1): 98-107.
-
[15] Caroll KC, JS Butel, SA Morse, T Mietzner, B Detrick, TG Mitchell, JH McKerrow, J A Sakanari. 2016. Jawetz, Melnick, & Adelberg’s Medical Microbiology, 27th Edition. McGraw-Hill, New York, Amerika Serikat.
-
[16] Sánchez S, AL Demain. 2015. Antibiotics: Current Innovations and Future Trends. Caister Academic Press, Norfolk, Inggris.
-
[17] Silva NMM, ISM Silva, RFS Pires, TLC Vasconcelos, MDM Viana, EA Campessato, LM
Conserva, EMM Rocha., EC Araujo, MLA Bastos. 2015. In Vitro Evaluation of Antimicrobial, Antioxidant, dan Larvicidal Activities from Extract of Zeyheria tuberlusa (Vell) Bur. (Biognoniace). Journal of Chemical and Pharmaceutical Research, 7: 319-328.
-
[18] Sunaryanto A, A, Mariyam. 1987. Occuraence of Pathogenic Bacteria Causing Luminescene in Penaeid Larvae in Indonesia Hatcheries. Bull. Brackhis Water Aqua. Devl. Centre, 8, 64-70.
Discussion and feedback